Abstract
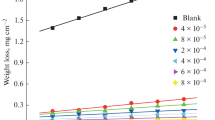
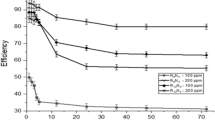
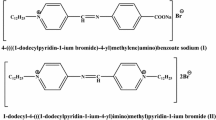
In the present work the corrosion inhibition of iron in 2 M HCl solution by amphoteric surfactants was studied. The techniques of measurements were (i) weight loss, (ii) linear polarization and (iii) electrochemical impedance spectroscopy. The investigated surfactants have the structure:

Fig. a
where, R = –C14H29(I), –C15H31(II), –C16H33(III), –C17H35(IV), –C18H37(V). These surfactants have a high inhibitory effect on the corrosion of iron in HCl solution. The inhibiting effect of these surfactants may take place through the blocking effect resulting from their adsorption on the metallic surface and hydrophobic effect. The inhibition efficiency increases according to the order: I < II < III < IV < V. This is due to the increase of the length of the alkyl group (–R) in the surfactants. The adsorption of these surfactants via their adsorption centers on the metallic surface obeyed the Frumkin adsorption isotherm. The presence of these surfactants in the corrosive solution increases the activation energy of the corrosion process with that order of inhibition efficiency.












Similar content being viewed by others
References
Pillali KC, Narayan R (1985) Corros Sci 23:151
Uhera J, Aramaki K (1991) J Electrochem Soc 138:3245
Jovancicevic V, Yang B, Bockris JO’M (1989) J Electrochem Soc 135:94
Bockris JO’M, Yang B (1991) J Electrochem Soc 138:2237
Tadros AB, Abdel-Nabey BA (1988) J Electroanal Chem 224:433
Abdel-Nabey BA, Elhoukhy A, Elgamal M, Mahmoud F (1986) Surf Coating Technol 27:325
Akust AA, Lorenz WJ, Mansfeld F (1982) Corros Sci 22:611
Hamouti B Aouniti A, Taleb M, Brighli M, Kertit S (1995) Corrosion 51:411
Sastri V, Perumareddi JR (1997) Corrosion 53:617
Clubley BG (1990) Chemical inhibitors for corrosion control. Royal Society of Chemistry, Cambridge
Uhlig HH, Revie RW (1985) Corrosion and corrosion control. John Wiley & Sons, New York, NY, p 263
Marsi MS, Barakat YF, El-Sheikh R, Hassan AM, Baraka A (1993) Werkst U Korros 44:304
Mansfeld F, Kending MW, Tsai S (1981) Corrosion 37:301
Mansfeld F, Kending MW, Tsai S (1982) Corrosion 38:570
Tsuru T, Haruyama S, Gijutsu B (1978) J Jpn Soc Corros Engn 27:573
Fouda AS, Moussa MN, Taha FI, Neanaa AI (1986) Corros Sci 26:719
Hajjaji N, Rico I, Srhiri A, Lattes A, Soufiaoui M, Ben Bachir A (1993) Corrosion 49:326
Koopal LK, Ralstan JJ (1986) Colloid Interface Sci 2:362
Perbani G, Rocchini G (1983) International conference on corrosion inhibitors. Dallas, May 1983, no 29
Ben Bachir A, Srhiri A, Derboli Y, Etman M, Lattas A (1991) J Appl Electrochem 21:261
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmoud, S.S. Corrosion inhibition of iron by amphoteric surfactants in hydrochloric acid solutions. J Mater Sci 42, 989–997 (2007). https://doi.org/10.1007/s10853-006-1389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1389-5