Abstract
Glass delamination, or the generation of glass flakes, continues to be an unwanted occurrence in the manufacture of parenteral (injectable) solutions and suspensions. In this root cause analysis study, advanced analytical tools including atomic force microscopy, environmental scanning electron microscopy, quantitative image analysis, and dynamic secondary ion mass spectroscopy (D-SIMS) showed significant differences in glass characteristics and performance. By observing the size and spatial arrangement of defects found on the interior surface of vials used as primary packaging for these products, in conjunction with the chemical changes that can occur to the glass because of product contact, a considerable amount of insight can be obtained into this phenomenon. Elemental depth profiling obtained by D-SIMS revealed that the interior vial surface was significantly altered by the presence of the parenteral solution, while another vial (manufactured by another vendor) was not. Although significant chemical changes can occur to the glass, the surface defect structure appears to be the dominant factor controlling the generation of glass flakes.















Similar content being viewed by others
Notes
Pharmaceutical nomenclature for borosilicate glass.
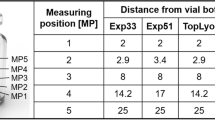
Upon heating, the ammonium sulfate decomposes. The gaseous sulfate reacts with the sodium in the glass, forming sodium sulfate, which is then removed when the vials are washed.
References
Ennis RD et al (2001) Pharm Dev Technol 6(3):393
Roseman TJ, Brown JA, Scothorn WW (1976) J Pharm Sci 65(1):22
Borchert SJ, Ryan MM (1989) J Parent Sci Technol 43(2):67–79
White WB (1992) In: Clark DE, Zoitos BK (eds) Corrosion of glass, ceramics and ceramic superconductors: principles, testing, characterization, and applications. Noyes Publications, Park Ridge, NJ, pp 2–28
Doremus RH (1967) In: Mitchell JW, DeVries RC (eds) Reactivity of solids. Wiley, New York, p 667
McIntyre NS, Strathdee GG, Phillips BF (1980) Surf Sci 100:71
Dimbley B (1953) J Pharm Pharmacol 5:969
Bacon FR, Raggon FC (1959) J Am Ceram Soc 42(4):199
Stevens HJ (1991) In: Schneider SJ (ed) Ceramics and glasses. ASM International, Materials Park, pp 394–401
Leadley SR et al (1998) Macromolecules 31(25):8957
Rossi A et al (2000) Surf Interface Anal 29(7):460
Swift AJ (1995) Mikrochim Acta 120(1–4):149
Vickerman JC, Briggs D (eds) (2001) ToF-SIMS: surface analysis by mass spectrometry. IMS Publications and Surface Spectra Limited, Chichester
Doremus RH (1995) J Mater Res 10(9):2379
Tomozawa H, Tomozawa M (1989) J Non-Crystal Solids 109:311
Doremus RH (1994) Glass science, 2nd edn. John Wiley & Sons Inc, New York, p 339
Adams PB (1977) Bull Parent Drug Assoc 31(5):213
Hair ML, Chapman ID (1966) J Am Ceram Soc 49(12):651
Davison RM, DeBold R, Johnson MJ (1987) In: Korb LJ, Olson BA (eds) ASM handbook. ASM International, Materials Park, OH, pp 547–566
Uhlig HH, Revie RW (1985) Corrosion and corrosion control, 3rd edn. John Wiley & Sons, New York, p 441
Fontana MG (1986) Corrosion engineering, 3rd edn. McGraw-Hill, New York, p 556
Fern S, McPhail DS, Oakley V (2004) Appl Surf Sci 231–232:510
Branda F et al (1999) Glass Technol 40(3):89
Gillies KJS, Cox A (1988) Glastech Ber 61(4):101
Schreiner M (1988) Glastech Ber 61(7):197
Rogers P, McPhail D, Ryan J (1993) Glass Technol 34(2):67
Acknowledgments
The authors would like to acknowledge the many participants in this study including: Dr. Dinesh Mishra, Dr. Heather Weimer, Mr. Eric Olsen, Ms. Sheryl Peoples, and Mr. David Crozier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iacocca, R.G., Allgeier, M. Corrosive attack of glass by a pharmaceutical compound. J Mater Sci 42, 801–811 (2007). https://doi.org/10.1007/s10853-006-0156-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0156-y