Abstract
Periodontal disease is characterized by a microbial infection and it is one of the major causes of teeth loss. The growth of pathogenic bacteria in oral cavity, such as Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), provides a favorable enviromment for the biofilm formation, which result in periodontal diseases. The development of new technologies to potentiate existing drugs, avoiding bacterial biofilms resistance and improving chemical stability is highly desirable. Here, we report the use of host–guest chemistry to enhance the activity of antibacterial doxycycline (DOX) in A. actinomycetemcomitans bacterial strain suspension and biofilm in vitro through complexation with hydroxypropyl-β-cyclodextrin (HPβCD) using different molar ratios of DOX/HPβCD. 2D 1H NMR confirmed the host–guest complexation of DOX and HPβCD. We assessed the colloidal characteristics of the complex DOX/HPβCD via Dynamic Light Scattering (DLS) and Zeta Potential (PZ). The mixing ratio 1:2 DOX/HPβCD significantly decreased the minimum inhibitory concentration (MIC) and improved efficacy against A. actinomycetemcomitans suspensions and biofilms, respectively, when compared to free DOX and other DOX/HPβCD complexes. Further, the interaction of different molar ratio proportions of DOX/HPβCD complex with bacterial membrane was demonstrated via Isothermal Titration Calorimetry (ITC). Thus, we suggested the enhanced efficacy of the DOX/HPβCD complexes, at molar ratio 1:2, is due the higher cyclodextrin ratio, which potentiate the interaction between drug and bacterial membrane through nonionic interactions, such as hydrogen bonding or other van der Waals interactions. Collectively, the development of these complexes enables increased efficacy against bacterial biofilms, which hold promise for the treatment of aggressive and non-responsive forms of periodontitis.








Similar content being viewed by others
Data availability
All data and material available upon request.
References
Tonetti, M.S., Greenwell, H., Kornman, K.S.: Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J. Periodontol. 89(Suppl 1), S159–S172 (2018)
Ramseier, C.A., Anerud, A., Dulac, M., Lulic, M., Cullinan, M.P., Seymour, G.J., Faddy, M.J., Burgin, W., Schatzle, M., Lang, N.P.: Natural history of periodontitis: disease progression and tooth loss over 40 years. J. Clin. Periodontol. 44, 1182–1191 (2017)
Houshmand, M., Holtfreter, B., Berg, M.H., Schwahn, C., Meisel, P., Biffar, R., Kindler, S., Kocher, T.: Refining definitions of periodontal disease and caries for prediction models of incident tooth loss. J. Clin. Periodontol. 39, 635–644 (2012)
Marcenes, W., Kassebaum, N.J., Bernabé, E., Flaxman, A., Naghavi, M., Lopez, A., Murray, C.J.L.: Global burden of oral conditions in 1990–2010: a systematic analysis. J. Dent. Res. 92, 592–597 (2013)
Papapanou, P.N.: The prevalence of periodontitis in the US: forget what you were told. J. Dent. Res. 91, 907–908 (2012)
Hajishengallis, G.: Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44 (2015)
Laine, M.L., Loos, B.G., Crielaard, W.: Gene polymorphisms in chronic periodontitis. Int J Dent. 2010, 1–22 (2010)
Deas, D.E., Moritz, A.J., Sagun, R.S.J., Gruwell, S.F., Powell, C.A.: Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontology 71, 128–139 (2016)
Larsson, L., Decker, A.M., Nibali, L., Pilipchuk, S.P., Berglundh, T., Giannobile, W.V.: Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 95, 255–266 (2016)
Habashneh, Al, R., Alsalman, W., Khader, Y.: Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: a randomized controlled clinical trial. J Periodontal Res. 50, 37–43 (2015)
Amano, A., Chen, C., Honma, K., Li, C., Settem, R.P., Sharma, A.: Genetic characteristics and pathogenic mechanisms of periodontal pathogens. Adv. Dent. Res. 26, 15–22 (2014)
Dhamecha, H.R.R., Jagwani, D., Rao, S., Jadhav, M., Shaikh, K., Puzhankara, S., Jalalpure, L.: S.: Local drug delivery systems in the management of periodontitis: a scientific review. J Control Release 307, 393–409 (2019)
Cortelli, J.R., Aquino, D.R., Cortelli, S.C., Roman-Torres, C.V.G., Franco, G.C.N., Gomez, R.S., Batista, L.H.B., Costa, F.O.: Aggregatibacter actinomycetemcomitans serotypes infections and periodontal conditions: a two-way assessment. Eur. J. Clin. Microbiol. Infect. Dis. 31, 1311–1318 (2012)
Slots, J., Ting, M.: Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 20, 82–121 (1999)
Mira, A., Simon-Soro, A., Curtis, M.A.: Role of microbial communities in the pathogenesis of periodontal diseases and caries. J. Clin. Periodontol. 44(Suppl 18), S23–S38 (2017)
Yoshida, A., Ennibi, O.-K., Miyazaki, H., Hoshino, T., Hayashida, H., Nishihara, T., Awano, S., Ansai, T.: Quantitative discrimination of Aggregatibacter actinomycetemcomitans highly leukotoxic JP2 clone from non-JP2 clones in diagnosis of aggressive periodontitis. BMC Infect. Dis. 12, 253–210 (2012)
Haubek, D., Ennibi, O.-K., Poulsen, K., Vaeth, M., Poulsen, S., Kilian, M.: Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 371, 237–242 (2008)
Penesyan, A., Nagy, S.S., Kjelleberg, S., Gillings, M.R., Paulsen, I.T.: Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes. 5, 34–14 (2019)
Høiby, N., Bjarnsholt, T., Givskov, M., Molin, S., Ciofu, O.: Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35, 322–332 (2010)
Bridier, A., Briandet, R., Thomas, V., Dubois-Brissonnet, F.: Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 27, 1017–1032 (2011)
Walker, C., Sedlacek, M.J.: An in vitro biofilm model of subgingival plaque. Oral Microbiol. Immunol. 22, 152–161 (2007)
Mah, T.F., O’Toole, G.A.: Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39 (2001)
Arciola, C.R., Campoccia, D., Speziale, P., Montanaro, L., Costerton, J.W.: Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 33, 5967–5982 (2012)
Heitz-Mayfield, L.J.A.: Systemic antibiotics in periodontal therapy. Aust. Dent. J. 54(Suppl 1), S96–S101 (2009)
Ghangurde, A.A., Ganji, K.K., Bhongade, M.L., Sehdev, B.: Role of chemically modified tetracyclines in the management of periodontal diseases: a review. Drug Res (Stuttg) 67, 258–265 (2017)
Preshaw, P.M., Hefti, A.F., Novak, M.J., Michalowicz, B.S., Pihlstrom, B.L., Schoor, R., Trummel, C.L., Dean, J., Van Dyke, T.E., Walker, C.B., Bradshaw, M.H.: Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. J. Periodontol. 75, 1068–1076 (2004)
Mohammad, A.R., Preshaw, P.M., Bradshaw, M.H., Hefti, A.F., Powala, C.V., Romanowicz, M.: Adjunctive subantimicrobial dose doxycycline in the management of institutionalised geriatric patients with chronic periodontitis. Gerodontology. 22, 37–43 (2005)
Novak, M.J., Johns, L.P., Miller, R.C., Bradshaw, M.H.: Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J. Periodontol. 73, 762–769 (2002)
Golub, L.M., McNamara, T.F., Ryan, M.E., Kohut, B., Blieden, T., Payonk, G., Sipos, T., Baron, H.J.: Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J. Clin. Periodontol. 28, 146–156 (2001)
Gueders, M.M., Bertholet, P., Perin, F., Rocks, N., Maree, R., Botta, V., Louis, R., Foidart, J.-M., Noel, A., Evrard, B., Cataldo, D.D.: A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem. Pharmacol. 75, 514–526 (2008)
Griffin, M.O., Fricovsky, E., Ceballos, G., Villarreal, F.: Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Cell Physiol. 299, C539–C548 (2010)
Madinier, I.M., Fosse, T.B., Hitzig, C., Charbit, Y., Hannoun, L.R.: Resistance profile survey of 50 periodontal strains of Actinobacillus actinomyectomcomitans. J. Periodontol. 70, 888–892 (1999)
Kulik, E.M., Lenkeit, K., Chenaux, S., Meyer, J.: Antimicrobial susceptibility of periodontopathogenic bacteria. J. Antimicrob. Chemother. 61, 1087–1091 (2008)
Ardila, C.M., Lopez, M.A., Guzman, I.C.: High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med Oral Patol Oral Cir Bucal. 15, e947–e951 (2010)
Feres, M., Haffajee, A.D., Goncalves, C., Allard, K.A., Som, S., Smith, C., Goodson, J.M., Socransky, S.S.: Systemic doxycycline administration in the treatment of periodontal infections (II). Effect on antibiotic resistance of subgingival species. J. Clin. Periodontol. 26, 784–792 (1999)
Chadha, V.S., Bhat, K.M.: The evaluation of doxycycline controlled release gel versus doxycycline controlled release implant in the management of periodontitis. J Indian Soc Periodontol. 16, 200–206 (2012)
Joshi, D., Garg, T., Goyal, A.K., Rath, G.: Advanced drug delivery approaches against periodontitis. Drug Delivery. 23, 363–377 (2014)
Pataro, A.L., Franco, C.F., Santos, V.R., Cortés, M.E., Sinisterra, R.D.: Surface effects and desorption of tetracycline supramolecular complex on bovine dentine. Biomaterials. 24, 1075–1080 (2003)
Denadai, ÂM.L., Teixeira, K.I., Santoro, M.M., Pimenta, A.M.C., Cortés, M.E., Sinisterra, R.D.: Supramolecular self-assembly of beta-cyclodextrin: an effective carrier of the antimicrobial agent chlorhexidine. Carbohydr. Res. 342, 2286–2296 (2007)
Franco, C.F., Pataro, A.L., Souza, E., Santos, L.C.R., Cortes, V.R., Sinisterra, M.E.: R.D.: In vitro effects of a chlorhexidine controlled delivery system. Artif. Organs 27, 486–491 (2003)
Guimarães, P.P.G., Tan, M., Tammela, T., Wu, K., Chung, A., Oberli, M., Wang, K., Spektor, R., Riley, R.S., Viana, C.T.R., Jacks, T., Langer, R., Mitchell, M.J.: Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J Control Release. 290, 75–87 (2018)
He, Z.-X., Wang, Z.-H., Zhang, H.-H., Pan, X., Su, W.-R., Liang, D., Wu, C.-B.: Doxycycline and hydroxypropyl-β-cyclodextrin complex in poloxamer thermal sensitive hydrogel for ophthalmic delivery. Acta Pharm. Sin. B. 1, 254–260 (2011)
National Committee for Clinical Laboratory Standards: Performance Standards for Antimicrobial Susceptibility Testing; Twenty First International Supplement. Wayne, PA: (2011)
Wiegand, I., Hilpert, K., Hancock, R.E.W.: Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 3, 163–175 (2008)
Burton, E., Yakandawala, N., LoVetri, K., Madhyastha, M.S.: A microplate spectrofluorometric assay for bacterial biofilms. J. Ind. Microbiol. Biotechnol. 34, 1–4 (2007)
Erriu, M., Genta, G., Tuveri, E., Orrù, G., Barbato, G., Levi, R.: Microtiter spectrophotometric biofilm production assay analyzed with metrological methods and uncertainty evaluation. Measurement. 45, 1083–1088 (2012)
Margarida Pereira, A., Cristina Abreu, A., Simões, M.: Action of kanamycin against single and dual species biofilms of Escherichia coli and Staphylococcus aureus. J. Microbiol. Res. 2, 84–88 (2012).
Bisson-Boutelliez, C., Fontanay, S., Finance, C., Kedzierewicz, F.: Preparation and physicochemical characterization of amoxicillin beta-cyclodextrin complexes. AAPS PharmSciTech 11, 574–581 (2010)
Abdelwahed, W., Degobert, G., Dubes, A., Parrot-Lopez, H., Fessi, H.: Sulfated and non-sulfated amphiphilic-beta-cyclodextrins: impact of their structural properties on the physicochemical properties of nanoparticles. Int. J. Pharm. 351, 289–295 (2008)
Denadai, ÂM.L., Santoro, M.M., Texeira, A.V., Sinisterra, R.D.: New insights regarding the cyclodextrin/AAS self-assembly: a molar ratio dependent system. Mater. Sci. Eng. C. 30, 417–422 (2010)
Lula, I., De Sousa, F.B., Denadai, ÂM.L., de Lima, G.F., Duarte, H.A., Mares Guia, dos, Faljoni-Alario, T.R., Santoro, A., de Camargo, M.M., Santos, A.C.M., dos, Sinisterra, R.A.S., R.D.: Interaction between bradykinin potentiating nonapeptide (BPP9a) and β-cyclodextrin: a structural and thermodynamic study. Mater. Sci. Eng. C. 32, 244–253 (2012)
Turnbull, W.B., Daranas, A.H.: On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J. Am. Chem. Soc. 125, 14859–14866 (2003)
Messner, M., Kurkov, S.V., Flavià-Piera, R., Brewster, M.E., Loftsson, T.: Self-assembly of cyclodextrins: the effect of the guest molecule. Mater. Sci. Eng. C. 408, 235–247 (2011)
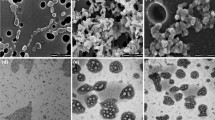
Teixeira, K.I.R., Araujo, P.V., Neves, B.R.A., Mahecha, G.A.B., Sinisterra, R.D., Cortes, M.E.: Ultrastructural changes in bacterial membranes induced by nano-assemblies beta-cyclodextrin chlorhexidine: SEM, AFM, and TEM evaluation. Pharm Dev Technol. 18, 600–608 (2013)
Consuegra, J., de Lima, M.E., Santos, D., Sinisterra, R.D., Cortes, M.E.: Peptides: beta-cyclodextrin inclusion compounds as highly effective antimicrobial and anti-epithelial proliferation agents. J. Periodontol. 84, 1858–1868 (2013)
Suárez, D.F., Consuegra, J., Trajano, V.C., Gontijo, S.M.L., Guimarães, P.P.G., Cortés, M.E., Denadai, ÂL., Sinisterra, R.D.: Structural and thermodynamic characterization of doxycycline/β-cyclodextrin supramolecular complex and its bacterial membrane interactions. Colloids Surf. B 118, 194–201 (2014)
Denadai, ÂM.L., de Oliveira, A.M., Daniel, I.M.P., Carneiro, L.A., Ribeiro, K.C., Beraldo, H., de O., da Costa, da Cunha, K.J.R., Cortés, V.C., Sinisterra, M.E.: R.D.: Chlorhexidine/losartan ionic pair binding and its nanoprecipitation: physico-chemical characterisation and antimicrobial activity. Supramol. Chem. 24, 204–212 (2012)
de Carvalho, F.G., Magalhães, T.C., Teixeira, N.M., Gondim, B.L.C., Carlo, H.L., Santos, dos, R.L., de Oliveira, A.R., Denadai, A.M.L.: Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C. 104, 109885 (2019)
Denadai, ÂM.L., Da Silva, J.G., Guimarães, P.P.G., Gomes, L.B.S., Mangrich, A.S., de Rezende, E.I.P., Daniel, I.M.P., Beraldo, H., Sinisterra, R.D.: Control of size in losartan/copper(II) coordination complex hydrophobic precipitate. Mater. Sci. Eng. C. 33, 3916–3922 (2013)
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329, 1–11 (2007)
Wingender, J., Neu, T.R., Flemming, H.-C.: What are bacterial extracellular polymeric substances? In: Microbial Extracellular Polymeric Substances, pp. 1–19. Springer, Berlin (1999)
Eastoe, J., Dalton, J.S.: Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv. Colloid Interface. Sci. 85, 103–144 (2000)
Acknowledgements
We are grateful for CNPq, CAPES, FAPEMIG, INCT/Nanobiofar that support this investigation. The authors also thank Professor Gustavo Menezes, Immunobiophotonics Lab, Universidade Federal de Minas Gerais (UFMG) for technical support and constructive advice.
Funding
CNPq, CAPES, FAPEMIG, INCT/Nanobiofar that support this investigation.
Author information
Authors and Affiliations
Contributions
PPGG, ACM, MEC and RDS conceived the ideas, designed the experiments, interpreted the data and wrote the manuscript. PPGG, ACM, KIRT, AMLD, and RAF conducted the experiments and analyzed the data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Consent for publication
All authors have read and approved this version of the article. The author signs for and accepts responsibility for releasing this material on behalf of any and all co-authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Guimarães, P.P.G., de Menezes, A.C., Teixeira, K.I.R. et al. Enhanced efficacy against bacterial biofilms via host:guest cyclodextrin‐doxycycline inclusion complexes. J Incl Phenom Macrocycl Chem 99, 197–207 (2021). https://doi.org/10.1007/s10847-020-01041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01041-7