Abstract
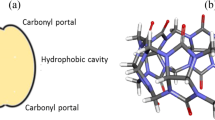
The intention of the study is to find the nature of interactions that exist in inclusion complexes formed between the steroids, nandrolone, and testosterone with cucurbit[n]urnils (n = 7 and 8) host, using density functional theory incorporated with empirical dispersion correction. Upon encapsulation, nandrolone caused a larger geometrical distortion in cucurbit[8]uril geometry, while testosterone inclusion complex is formed with a larger number of intermolecular hydrogen bonds. The molecular electrostatic potential examination shows that the positive potential observed on the eight-membered ring in CB7 got reduced upon encapsulation, while on the nandrolone the negative potential on carbonyl unit has increased. AIM analysis shows that in inclusion complexes, the observed electron density are higher for the interactions between the oxygen atoms of carbonyl fringe of cucurbituril molecule and the steroid molecules. The NCI isosurface of nandrolone@CB7 has green patches in between the nandrolone and cucurbituril molecule, evenly distributed. In the testosterone@CB7, along with the green patches, red patches, due to the steric crowding between the testosterone and cucurbit[7]uril, were observed. The energy decomposition analysis parameters show that Pauli’s repulsive term was highest for nandrolone@CB7. When testosterone is the guest, repulsive energy was found to be larger than nandrolone guest. From the above interference, it can be confirmed that the steric hindrance that arises during the interaction of testosterone with CB7 reduces the stability of the complex, and the nandrolone best fit inside the CB7 cavity with the combination of hydrogen bonding and weak van der Waals bonding as intermolecular interactions.





Similar content being viewed by others
References
Schneider, H.J., Schiestel, T., Zimmermann, P.: Host-guest supramolecular chemistry. 34. the incremental approach to noncovalent interactions: coulomb and van der Waals effects in organic ion pairs. J. Am. Chem. Soc. 114, 7698–7703 (1992)
Puttreddy, R., Beyeh, N.K., Ras, R.H.A., Rissanen, K.: Host-guest complexes of C-ethyl-2-methylresorcinarene and aromatic N,Nʹ-dixodes. Chemistryopen 6, 417–423 (2017)
Webber, M.J., Langer, R.: Drug delivery by supramolecular design. Chem. Soc. Rev. 46, 6600–6620 (2017)
Moussa, Y.E., Ong, Y.Q.E., Perry, J.D., Cheng, Z., Kayser, V., Cruz, E., Kim, R.R., Sciortino, N., Wheate, N.J.: Demonstration of in vitro host-guest complex formation and safety of para-sulfonatocalix[8]arene as a delivery vechicle for two antibiotic drugs. J. Pharm. Sci. https://doi.org/10.1016/j.xphs.2018.08.016 (2018)
Al-Dubaili, N., El-Tarabily, K., Shaleh, N.: Host-guest complexes of imazalil with cucurbit[8]uril and β-cyclodextrin and their effect on plant pathogenic fungi. Sci. Rep. 8, 2839–2849 (2018)
Ghosh, R., Ekka, D., Rajbanshi, B., Yasmin, A., Roy, N.M.: Synthesis, characterization of 1-butyl-4-methylpyridinium lauryl sulfate and its inclusion phenomenon with β-cyclodextrin for enhanced applications. Colloid. Surf. A. 548, 206–217 (2018)
Spenst, P., Sieblist, A., Wůrthner, F.: Perylene bisimide cyclophanes with high binding affinity for large planar polycyclic aromatic hydrocarbons: Host-guest complexation versus self-encapsulation of side arms. Chem. Eur. J. 23, 1667–1675 (2017)
Fahmy, S.A., Ponte, F., Abd El-Rahman, M.K., Russo, N., Sicilia, E., Shoeib, T.: Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug deliver. Spectrochim. Acta. A. 193, 528–536 (2018)
Tan, L.-L., Zhang, Y., Li, B., Wang, K., Zhang, X.-A., Tao, S., Yang, Y.: Y.-W.: Selective recognition of solvent molecules in solution and the solid state by 1,4-dimethoxypillar[5]arene driven by attractive forces. New. J. Chem. 38, 845–851 (2014)
Rekharsky, M.V., Mori, T., Yang, C., Ko, Y.H., Selvapalam, N., Kim, H., Sobransingh, D., Kaifer, A.E., Liu, S., Lsaacs, L., Chen, W., Moghaddam, S., Gilson, M.K., Kim, K., Inoue, Y.: A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy-entropy compensation. Proc. Natl. Acad. Sci. USA. 104, 20732–20742 (2007)
Aryal, G.H., Vik, R., Assaf, K.I., Hunter, K.W., Huang, L., Jayawickramarajah, J.: Structural effects on guest binding in cucurbit[8]uril-perylneneimide host-guest complexes. ChemistrySelect 3, 4699–4704 (2018)
Yin, H., Huang, Q., Zhao, W., Bardelang, D., Siri, D., Chen, X., Lee, S.M.Y., Wang, R.: Supramolecular encapsulation and bioactivity modulation of a halonium ion by cucurbit[n]uril (n = 7, 8). J. Org. Chem. 83, 4882–4887 (2018)
Barooah, N., Khurana, R., Bhasikuttan, A.C., Mohanty, J.: Stimuli-responsive supra-biomolecular nanoassemblies of cucurbit[7]uril with bovine serum albumin: Drug delivery and sensor applications. Isr. J. Chem. 58, 276–285 (2018)
Danylyuk, O., Butkiewicz, H., Coleman, A.W., Suwinska, K.: Host-guest complexes of local anesthetics with cucurbit[6]uril and para-sulphonatocalix[8]arene in solid state. J. Mol. Struct. 1150, 28–36 (2017)
Hostaš, J., Sigwalt, D., Šekutor, M., Ajani, H., Dubecky, M., Řezáč, J., Zavliji, P.Y., Cao, L., Wohlschalger, C., Mlinarič-Majerski, K., Issacs, L.I., Glaser, R., Hobza, P.: A nexus between theory and experiment: non-empirical quantum mechanical computational methodology applied to cucurbit[n]uril.guest binding interactions. Chem. A. Eur. J. 48, 17226–17238 (2016)
Hassanzadeh, K., Akhtari, K., Esmaeili, S.S., Vaziri, A., Zamani, H.: Encapsulation of thiotepa and altretamine as neutrotoxic anticancer drugs in cucurbit[n] uril (n = 7,8) nanocapsules: a DFT study. J. Theor. Comput. Chem. 15, 1650056 (2016)
Shewale, M.N., Lande, D.N., Gejji, S.P.: Encapsulation of benzimidazole derivatives within cucurbit[7]uril: density functional investigations. J. Mol. Liq. 216, 309–317 (2016)
Poša, M., Popović, K.: Structure-property relationships in sodium muricholate derivative (bile salts) micellization: the effect of conformation of steroid skeleton on hydrophobicity and micelle formation-pattern recognition and potential membranoprotective properties. Mol. Pharam. 14, 3343–3355 (2017)
Bai, G., Sheng, J., Wang, Y., Wu, H., Zhao, Y., Zhuo, K., Bastos, M.: Interaction between a hydrophobic rigid face and flexible alkyl tail: thermodynamics of self-assembling of sodium cholate and SDS. J. Chem. Theormodyn. 100, 131–139 (2016)
Assaf, K.I., Florea, M., Antony, J., Henriksen, N.M., Yin, J., Hansen, A., Qu, Z., Sure, R., Klapstein, D., Gilson, M.K., Grimme, S., Nau, W.M.: HYDROPHOBE challenge: a joint experimental and computational study on the host-guest binding of hydrocarbons to cuubiturils, allowing explicit evaluation of guest hydration free-energy contributions. J. Phys. Chem. B. 121, 11144–11162 (2017)
Gamal-Eldin, M.A., Macartney, D.H.: Cucurbit[7]uril host-guest complexations of steroidal neuromuscular blocking agents in aqueous solution. Can. J. Chem. 92, 243–249 (2014)
Lazar, A.I., Biedermann, F., Mustafina, K.R., Assaf, K.I., Hennig, A., Nau, W.M.: Nanomolar binding of steroids to cucurbit[n]urils: selectivity and applications. J. Am. Chem. Soc. 138, 13022–13029 (2016)
Grimme, S.: Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 27, 1787–1799 (2006)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E. Jr., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Star-overov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Strat-mann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman J.B, Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian G09, Revision D.01. Gaussian, Inc., Wallingford (2010)
Simon, S., Duran, M., Dannenberg, J.J.: How does basis set superposition error change the potential surfaces for hydrogen bonded dimers? J. Chem. Phys. 105, 11024–11031 (1996)
Klamt, A., Jonas, V., Burger, T., Lohrenz, J.C.W.: Refinement and Parameterization of COSMO-RS. J. Phys. Chem. A 102, 5074–5085 (1998)
Bulat, F.A., Toro-Labbė, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16, 1679–1691 (2010)
Venkataramanan, N.S., Suvitha, A.: Theoretical investigation of the binding of nucleobases to cucurbiturils by disperstion corrected DFT approaches. J. Phys. Chem. B. 121, 4733–4744 (2017)
Venkataramanan, N.S., Suvitha, A., Kawazoe, Y.: Unravelling the nature of the binding of cubane and substituted cubanes within cucurbiturils: A DFT and NCI study. J. Mol. Liq. 260, 18–19 (2018)
Venkataramanan, N.S., Suvitha, A.: Encapsulation of sulfur, oxygen, and nitrogen mustards by cucurbiturils: a DFT study. J. Incl. Phenom. Macrocycl. Chem. 83, 387–400 (2015)
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
Zhurko, G.A., Zhurko, D.A.: Chemcraft. http://www.chemcraftprog.com/
te Velde, G., Bickelhaupt, F.M., Baerends, E.J., Guerra, C.F., van Gisbergen, S.J.A., Snijders, J.G., Ziegler, T.: Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001)
Mohanty, B., Suvitha, A., Venkataramanan, N.S.: Piperine encapsulation within cucurbit[n]uril (n = 6, 7): a combined experimental and density functional study. ChemistrySelect 3, 1933–1941 (2018)
Assaf, K.I., Nau, W.M.: Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 44, 394–418 (2015)
Venkataramanan, N.S., Suvitha, A.: Structure, electronic, inclusion complex formation behavior and spectral properties of pillarplex. J. Incl. Phenom. Macrocycl. Chem. 88, 53–67 (2017)
Padmanabhan, J., Parthasarathi, R., Subramanian, V., Chattaraj, P.K.: Electrophilicity based charge transfer descriptor. J. Phys. Chem. A 111, 1358–1361 (2007)
Gupta, K., Giri, S., Chattaraj, P.K.: Charge-based DFT descriptors for diels-alder reactions. J. Phys. Org. Chem. 26, 187–193 (2013)
Marama, N.L., Casassa, S.M., Sambrano, J.R.: Adsorption of NH3 with different coverages on single-walled ZnO nanotube: DFT and QTAIM study. J. Phys. Chem. C 121, 8109–8119 (2017)
Hussain, M.A., Soujanya, Y., Sastry, G.N.: Computational design of functionalized imidazolate linkers of zeolitic imidazolate frameworks for enhanced CO2 adsorption. J. Phys. Chem. C 119, 23607–23618 (2015)
Venkataramanan, N.S., Suvitha, A.: Nature of bonding and cooperativity in linear DMSO clusters: a DFT, AIM and NCI analysis. J. Mol. Graph. Model. 81, 50–59 (2018)
Ziegler, T., Rauk, A.: On the calculation of bonding energies by the Hartree–Fock slater method. Theor. Chim. Acta. 45, 1–10 (1977)
Funding
Funding was provided by Science and Engineering Research Board (Grant No: EMR-II-SB/S1/PC-047/2013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suvitha, A., Souissi, M., Sahara, R. et al. Deciphering the nature of interactions in nandrolone/testosterone encapsulated cucurbituril complexes: a computational study. J Incl Phenom Macrocycl Chem 93, 183–192 (2019). https://doi.org/10.1007/s10847-018-0869-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0869-y