Abstract
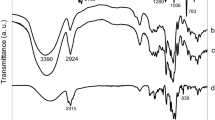
The genus Lantana is widely used in folk medicine because its essential oil has antibacterial, antifungal and repellent activity. However, its thermal instability and low solubility in water reduce its technological application. In this work, an inclusion complex was prepared consisting of the essential oil of Latana camara L. (LCEO) and β-cyclodextrin (β-CD). The complex was characterizated by vibrational spectroscopy (Raman and FTIR spectroscopy), differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The optimization of the ratio of the LCEO and β-CD in the inclusion complex was determined by gas chromatography coupled to mass spectrometry (GC–MS); it was inferred that the ratio of guest–host = 6:94 (m:m) was the optimal ratio. Shifts in some peak positions of the vibrations modes in Raman spectra of L. camara and β-CD provided clearer and better evidence of inclusion complex formation than infrared spectroscopy did. Results of DSC and XRD characterization of inclusion complex are in good agreement with Raman results.
Graphical Abstract
Formation of the inclusion complex between β-CD and LCEO as well as its characterization by different analytical techniques such as infrared spectroscopy and Raman spectroscopy, DSC, XRD and GC–MS.







Similar content being viewed by others
References
Patel, S.: A weed with multiple utility: Lantana camara. Rev. Environ. Sci. Biotechnol. 10, 341–351 (2011). https://doi.org/10.1007/s11157-011-9254-7
Bouda, H., Tapondjou, L.A., Fontem, D.A., Gumedzoe, M.Y.D.: Effect of essential oils from leaves of Ageratum conyzoides, Lantana camara and Chromolaena odorata on the mortality of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 37, 103–109 (2001). https://doi.org/10.1016/S0022-474X(00)00011-4
Silva, R.A.D.: Pharmacopeia Brasileira. Companhia Editora Nacional, Rio de Janeiro (1929)
Brandão, M.G.L., Zanetti, N.N.S., Oliveira, G.R.R., Goulart, L.O., Monte-Mor, R.L.M.: Other medicinal plants and botanical products from the first edition of the Brazilian Official Pharmacopoeia. Rev. Bras. Farmacogn. 18, 127–134 (2008). https://doi.org/10.1590/S0102-695X2008000100022
Alitonou, G., Avlessi, F., Bokossa, I., Ahoussi, E., Dangou, J., Sohounhloué, D.C.K.: Composition chimique et activités biologiques de l’huile essentielle de Lantana camara Linn. Comptes Rendus Chim. 7, 1101–1105 (2004). https://doi.org/10.1016/j.crci.2003.11.017
Deena, M.J., Thoppil, J.E.: Antimicrobial activity of the essential oil of Lantana camara. Fitoterapia 71, 453–455 (2000). https://doi.org/10.1016/S0367-326X(00)00140-4
Sousa, E.O., Silva, N.F., Rodrigues, F.F.G., Campos, A.R., Lima, S.G., Costa, J.G.M.: Chemical composition and resistance-modifying effect of the essential oil of Lantana camara Linn. Pharmacogn. Mag. 6, 79–82 (2010). https://doi.org/10.4103/0973-1296.62890
Benites, J., Moiteiro, C., Miguel, G., Rojo, L., López, J., Venâncio, F., Ramalho, L., Feio, S., Dandlen, S., Casanova, H., Torres, I.: Composition and biological activity of the essential oil of peruvian Lantana camara. J. Chil. Chem. Soc. 54, 379–384 (2009). https://doi.org/10.4067/S0717-97072009000400012
Costa, J.G.M., Sousa, E.O., Rodrigues, F.F.G., De Lima, S.G., Braz-Filho, R.: Composição química e avaliação das atividades antibacteriana e de toxicidade dos óleos essenciais de Lantana camara L. e Lantana sp. Braz. J. Pharmacogn. 19, 710–714 (2009). https://doi.org/10.1590/S0102-695X2009000500010
Costa, J.G.M., Rodrigues, F.F.G., Sousa, E.O., Junior, D.M.S., Campos, A.R., Coutinho, H.D.M., De Lima, S.G.: Composition and larvicidal activity of the essential oils of lantana camara and lantana montevidensis. Chem. Nat. Compd. 46, 313–315 (2010). https://doi.org/10.1007/s10600-010-9601-x
Heise, H.M., Kuckuk, R., Bereck, A., Riegel, D.: Infrared spectroscopy and Raman spectroscopy of cyclodextrin derivatives and their ferrocene inclusion complexes. Vib. Spectrosc. 53, 19–23 (2010). https://doi.org/10.1016/j.vibspec.2010.01.012
Zoubiri, S., Baaliouamer, A.: GC and GC/MS analyses of the Algerian Lantana camara leaf essential oil: effect against Sitophilus granarius adults. J. Saudi Chem. Soc. 16, 291–297 (2012). https://doi.org/10.1016/j.jscs.2011.01.013
Zoubiri, S., Baaliouamer, A.: Larvicidal activity of two Algerian Verbenaceae essential oils against Culex pipiens. Vet. Parasitol. 181, 370–373 (2011). https://doi.org/10.1016/j.vetpar.2011.04.033
Kubo, A., Lunde, C.S., Kubo, I.: Antimicrobial activity of the olive oil flavor compounds. J. Agric. Food Chem. 43, 1629–1633 (1995). https://doi.org/10.1021/jf00054a040
Lu, J.J., Dang, Y.Y., Huang, M., Xu, W.S., Chen, X.P., Wang, Y.T.: Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae: a review. J. Ethnopharmacol. 143, 406–411 (2012). https://doi.org/10.1016/j.jep.2012.07.009
Ghelardini, C., Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., Bartolini, A.: Local anaesthetic activity of β-caryophyllene. Farmaco 56, 387–389 (2001). https://doi.org/10.1016/S0014-827X(01)01092-8
Aguiara, U.N., De Lima, S.G., Rocha, M.S., De Freitas, R.M., Oliveira, T.M., Silva, R.M., Moura, L.C.B., De Almeidab, L.T.G.: Preparação e caracterização do complexo de inclusão do óleo essencial de croton zehntneri com b-ciclodextrina. Quim. Nova. 37, 50–55 (2014). https://doi.org/10.1590/S0100-40422014000100010
Lyra, M.A.M., Alves, L.D.S., Fontes, D.A.F., Soares-Sobrinho, J.L., Rolim-Neto, P.J.: Ferramentas analíticas aplicadas à caracterizaçã o de complexos de inclusão fármaco-ciclodextrina. Rev. Ciencias Farm. Basica Apl. 31, 117–124 (2010)
Salústio, P.J., Feio, G., Figueirinhas, J.L., Pinto, J.F., Cabral Marques, H.M.: The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur. J. Pharm. Biopharm. 71, 377–386 (2009). https://doi.org/10.1016/j.ejpb.2008.09.027
Wang, J., Cao, Y., Sun, B., Wang, C.: Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 127, 1680–1685 (2011). https://doi.org/10.1016/j.foodchem.2011.02.036
Abbehausen, C., Formiga, A.L.B., Sabadini, E., Yoshida, I.V.P.: A-βcyclodextrin/siloxane hybrid polymer: synthesis, characterization and inclusion complexes. J. Braz. Chem. Soc. 21, 1867–1876 (2010). https://doi.org/10.1590/S0103-50532010001000011
Britto, M.A.F.O., Nascimento, C.S., Dos Santos, H.F.: Análise estrutural de ciclodextrinas: Um estudo comparativo entre métodos teóricos clássicos e quânticos. Quim. Nova. 27, 882–888 (2004). https://doi.org/10.1590/S0100-40422004000600008
Fernandes, L.P., Oliveira, W.P., Sztatisz, J., Szilágyi, I.M., Novák, C.: Solid state studies on molecular inclusions of Lippia sidoides essential oil obtained by spray drying. J. Therm. Anal. Calorim. 95, 855–863 (2009). https://doi.org/10.1007/s10973-008-9149-1
Waleczek, K.J., Cabral Marques, H.M., Hempel, B., Schmidt, P.C.: Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur. J. Pharm. Biopharm. 55, 247–251 (2003). https://doi.org/10.1016/S0939-6411(02)00166-2
Wang, Y., Jiang, Z.-T., Li, R.: Complexation and molecular microcapsules of Litsea cubeba essential oil with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 228, 865–873 (2009). https://doi.org/10.1007/s00217-008-0999-3
Zhan, H., Jiang, Z.-T., Wang, Y., Li, R., Dong, T.-S.: Molecular microcapsules and inclusion interactions of eugenol with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 227, 1507–1513 (2008). https://doi.org/10.1007/s00217-008-0873-3
Jiang, S., Li, J.-N., Jiang, Z.-T.: Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil. Eur. Food Res. Technol. 230, 543–550 (2010). https://doi.org/10.1007/s00217-009-1192-z
Rosa, R.-D.L., Cevallos-Ferriz, R.A., Silva-Pineda, S.R.S.A.: Paleobiological implications of Campanian coprolites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 142, 231–254 (1998). https://doi.org/10.1016/S0031-0182(98)00052-2
Reineccius, T.A., Reineccius, G.A., Peppard, T.L.: The effect of solvent interactions on α-, β-, and γ-cyclodextrin/flavor molecular inclusion complexes. J. Agric. Food Chem. 53, 388–392 (2005). https://doi.org/10.1021/jf0488716
de Oliveira, V.E., Almeida, E.W.C., Castro, H.V., Edwards, H.G.M., Dos Santos, H.F., de Oliveira, L.F.C.: Carotenoids and β-cyclodextrin inclusion complexes: Raman spectroscopy and theoretical investigation. J. Phys. Chem. A 115, 8511–8519 (2011). https://doi.org/10.1021/jp2028142
de Lima, S.G., Neto, J.M.M., Lopes Citó, A.M.G., da Costa, J.G.M., Reis, F.A.M.: Monoterpenes, sesquiterpenes and fatty acids from julocroton triqueter (Euphorbiaceae) from Ceara-Brazil. J. Chil. Chem. Soc. 54, 55–57 (2009). https://doi.org/10.4067/S0717-97072009000100013
Van Den Dool, H., Dec. Kratz, P.: A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 11, 463–471 (1963). https://doi.org/10.1016/S0021-9673(01)80947-X
Adams, R.P.: Identification of essential oil components by gas chromatography/massspectrometry. Allured Pub. Corp, Carol Stream (2007)
Medeiros, L.B.P., Rocha, MdosS., de Lima, S.G., de Sousa Júnior, G.R., Lopes Citó, A.M.G., da Silva, D., Lopes, J.A.D., Moura, D.J., Saffi, J., Mobin, M., da Costa, J.G.M.: Chemical constituents and evaluation of cytotoxic and antifungal activity of Lantana camara essential oils. Rev. Bras. Farmacogn. 22, 1259–1267 (2012). https://doi.org/10.1590/S0102-695X2012005000098
Bhandari, B.R., Arcy, B.R.D., Le, L., Bich, T.: Lemon oil to β-cyclodextrin ratio effect on the inclusion efficiency of β-cyclodextrin and the retention of oil volatiles in the complex. Analysis 8561, 1494–1499 (1998). https://doi.org/10.1021/jf970605n
Harangi, J., Nánási, P.: Measurement of the essential oil in inclusion complexes with cyclodextrin by means of capillary gas chromatography. Anal. Chim. Acta. 156, 103–109 (1984). https://doi.org/10.1016/S0003-2670(00)85541-5
Sonibare, O., Effiong, I.: African journal of biotechnology. Academic Journals (2002)
Hernández, T., Canales, M., Avila, J.G., García, A.M., Martínez, A., Caballero, J., De Vivar, R., Lira, A.R.: Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae). J. Ethnopharmacol. 96, 551–554 (2005). https://doi.org/10.1016/j.jep.2004.09.044
Randrianalijaona, J.A., Ramanoelina, P.A.R., Rasoarahona, J.R.E., Gaydou, E.M.: Seasonal and chemotype influences on the chemical composition of Lantana camara L.: essential oils from Madagascar. Anal. Chim. Acta. 545, 46–52 (2005). https://doi.org/10.1016/j.aca.2005.04.028
Baranska, M., Schulz, H., Walter, A., Rösch, P., Quilitzsch, R., Lösing, G., Popp, J.: Investigation of eucalyptus essential oil by using vibrational spectroscopy methods. Vib. Spectrosc. 42, 341–345 (2006). https://doi.org/10.1016/j.vibspec.2006.08.004
Misra, L., Laatsch, H.: Triterpenoids, essential oil and photo-oxidative 28→13-lactonization of oleanolic acid from Lantana camara. Phytochemistry 54, 969–974 (2000)
Ngassoum, M.B., Yonkeu, S., Jirovetz, L., Buchbauer, G., Schmaus, G., Hammerschmidt, F.J.: Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 14, 245–250 (1999)
Veiga, F., Pecorelli, C., Ribeiro, L.: As ciclodextrinas em tecnologia farmacêutica. MinervaCoimbra, Coimbra (2006)
Hǎdǎrugǎ, N.G., Hǎdǎrugǎ, D.I., Pǎunescu, V., Tatu, C., Ordodi, V.L., Bandur, G., Lupea, A.X.: Thermal stability of the linoleic acid/α- and β-cyclodextrin complexes. Food Chem. 99, 500–508 (2006). https://doi.org/10.1016/j.foodchem.2005.08.012
Schulz, H., Baranska, M.: Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 43, 13–25 (2007). https://doi.org/10.1016/j.vibspec.2006.06.001
Schulz, H., Quilitzsch, R., Krüger, H.: Rapid evaluation and quantitative analysis of thyme, origano and chamomile essential oils by ATR-IR and NIR spectroscopy. J. Mol. Struct. 661–662, 299–306 (2003). https://doi.org/10.1016/S0022-2860(03)00517-9
Li, W., Lu, B., Chen, F., Yang, F., Wang, Z.: Host–guest complex of cypermethrin with β-cyclodextrin: a spectroscopy and theoretical investigation. J. Mol. Struct. 990, 244–252 (2011). https://doi.org/10.1016/j.molstruc.2011.01.053
Li, W., Lu, B., Sheng, A., Yang, F., Wang, Z.: Spectroscopic and theoretical study on inclusion complexation of beta-cyclodextrin with permethrin. J. Mol. Struct. 981, 194–203 (2010). https://doi.org/10.1016/j.molstruc.2010.08.008
Egyed, O.: Spectroscopic studies on β-cyclodextrin. Anal. Chim. Acta. 240, 225–227 (1990). https://doi.org/10.1016/0924-2031(90)80041-2
Schulz, H., Özkan, G., Baranska, M., Krüger, H., Özcan, M.: Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 39, 249–256 (2005). https://doi.org/10.1016/j.vibspec.2005.04.009
Larkin, P.: Infrared and Raman spectroscopy: principles and spectral interpretation. Elsevier, Amsterdam (2011)
Stancanelli, R., Ficarra, R., Cannavà, C., Guardo, M., Calabrò, M.L., Ficarra, P., Ottanà, R., Maccari, R., Crupi, V., Majolino, D., Venuti, V.: UV-VIS and FTIR-ATR characterization of 9-fluorenon-2-carboxyester/(2-hydroxypropyl)-β-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 47, 704–709 (2008). https://doi.org/10.1016/j.jpba.2008.02.018
Iliescu, T., Baia, M., Miclăuş, V.: A Raman spectroscopic study of the diclofenac sodium–β-cyclodextrin interaction. Eur. J. Pharm. Sci. 22, 487–495 (2004). https://doi.org/10.1016/j.ejps.2004.05.003
Seidler-Lozykowska, K., Baranska, M., Baranski, R., Krol, D.: Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. J. Agric. Food Chem. 58, 5271–5275 (2010). https://doi.org/10.1021/jf100298z
Siatis, N.G., Kimbaris, A.C., Pappas, C.S., Tarantilis, P.A., Daferera, D.J., Polissiou, M.G.: Rapid method for simultaneous quantitative determination of four major essential oil components from Oregano (Oreganum sp.) and Thyme (Thymus sp.) using FT-Raman spectroscopy. J. Agric. Food Chem. 53, 202–206 (2005). https://doi.org/10.1021/jf048930f
Daferera, D.J., Tarantilis, P.A., Polissiou, M.G.: Characterization of essential oils from lamiaceae species by fourier transform Raman spectroscopy. J. Agric. Food Chem. 50, 5503–5507 (2002)
Daferera, D., Pappas, C., Tarantilis, P.A., Polissiou, M.: Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 77, 511–515 (2002). https://doi.org/10.1016/S0308-8146(01)00382-X
Fini, A., Ospitali, F., Zoppetti, G., Puppini, N.: ATR/Raman and fractal characterization of HPBCD/progesterone complex solid particles. Pharm. Res. 25, 2030–2040 (2008). https://doi.org/10.1007/s11095-008-9593-4
Lamcharfi, E., Kunesch, G., Meyer, C., Robert, B.: Using FT-IR and Raman spectroscopy. Spectroscopy 51, 1861–1870 (1995)
Acknowledgements
The authors would like to acknowledge the UFPI and CNPq for financial support and FISMAT and LAPETRO for the analyzes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, M.d.S., de Lima, S.G., Viana, B.C. et al. Characterization of the inclusion complex of the essential oil of Lantana camara L. and β-cyclodextrin by vibrational spectroscopy, GC–MS, and X-ray diffraction. J Incl Phenom Macrocycl Chem 91, 95–104 (2018). https://doi.org/10.1007/s10847-018-0799-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0799-8