Abstract
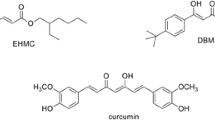
The water-soluble inclusion complexes of curcumin, 2-ethylhexyl-4-methoxycinnamate (EHMC) and 4-tert-butyl-4′-methoxydibenzoylmethane (DBM) with gamma-cyclodextrin polymer (pyCD) were successfully prepared and characterized by 1H-NMR, IR and UV–Vis spectroscopies. The water-solubility of EHMC–pyCD, DBM–pyCD and curcumin–pyCD was dramatically increased because of the water-soluble pyCD. The mole ratio of gamma-CD in pyCD to EHMC, DBM and curcumin were carried out as 1:1. The photostability of all inclusion complexes was investigated in water and ethylene glycol and compared with free active ingredients. It was found that the photostability of EHMC–pyCD was greatly enhanced whereas those of DBM–pyCD and curcumin–pyCD in water were decreased due to the acceleration of the photodegradation reaction inside the gamma-CD cavity. These results were able to be used as a solubilizer for cosmetic and also pharmaceutical applications.








Similar content being viewed by others
References
Agar, N.S., Halliday, G.M., Barnetson, R.S., Ananthaswamy, H.N., Wheeler, M., Jones, A.M.: The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc. Natl Acad. Sci. U. S. A. 101, 4954–4959 (2004)
Matsumura, Y., Ananthaswamy, H.N.: Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 195, 298–308 (2004)
Kockler, J., Oelgemöller, M., Robertson, S., Glass, B.D.: Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 13, 91–110 (2012)
Pattanaargson, S., Munhapol, T., Hirunsuphachot, P., Limpong, P.: Photoisomerization of octyl methoxycinnamate. J. Photochem. Photobiol. A Chem. 161, 269–274 (2004)
Mturi, G.J., Martincigh, B.S.: Photostability of the sunscreening agent 4-tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A Chem. 200, 410–420 (2008)
Kockler, J., Motti, C., Robertson, S., Oelgemöller, M., Glass, B.D.: HPLC Method for the Simultaneous Determination of the UV-Filters Butyl Methoxy Dibenzoylmethane and Octocrylene in the Presence of Their Photodegradants. Chromatographia. 76, 1721–1727 (2013)
Wang, Y.J., Pan, M.H., Cheng, A.L., Lin, L.I., Ho, Y.S., Hsieh, C.Y., Lin, J.K.: Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 15, 1867-1876 (1997)
Jahed, V., Zarrabi, A., Bordbar, A., Hafezi, M.S.: NMR (1H, ROESY) spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin and curcumin. Food Chem. 165, 241–246 (2014)
Ammala, A.: Biodegradable polymers as encapsulation materials for cosmetics and personal care markets, Int. J. Cosmet. Sci. 35, 113–124 (2013)
Yallapu, M.M., Jaggi, M., Chauhan, S.C.: β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surfaces B Biointerfaces. 79, 113–125 (2010)
Kurkov, S. V., Loftsson, T.: Cyclodextrins. Int. J. Pharm. 453, 167–180 (2013)
Anand, R., Manoli, F., Manet, I., Daoud-Mahammed, S., Agostoni, V., Gref, R., Monti, S.: β-Cyclodextrin polymer nanoparticles as carriers for doxorubicin and artemisinin: a spectroscopic and photophysical study. Photochem. Photobiol. Sci. 11, 1285-1292 (2012)
Scalia, S., Simeoni, S., Barbieri, A., Sostero, S.: Influence of hydroxypropyl-β-cyclodextrin on photo-induced free radical production by the sunscreen agent, butyl-methoxydibenzoylmethane. J. Pharm. Pharmacol. 54,1553–1558 (2002)
Suwannateep, N., Banlunara, W., Wanichwecharungruang, S.P., Chiablaem, K., Lirdprapamongkol, K., Svasti, J.: Mucoadhesive curcumin nanospheres: Biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J. Control. Release. 151, 176–182 (2011)
Scalia, S., Casolari, A., Iaconinoto, A., Simeoni, S.: Comparative studies of the influence of cyclodextrins on the stability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. J. Pharm. Biomed. Anal. 30, 1181–1189 (2002)
Liu, Y., Chen, G.-S., Chen, Y., Ding, F., Chen, J.: Cyclodextrins as carriers for cinchona alkaloids: a pH-responsive selective binding system. Org. Biomol. Chem. 3, 2519–23 (2005)
Ciobanu, a., Mallard, I., Landy, D., Brabie, G., Nistor, D.: Fourmentin, S. Fourmentin, Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr. Polym. 87, 1963–1970 (2012)
Renard, E., Deratani, a., Volet, G., Sebille, B.: Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 33, 49–57 (1997)
Zhang, W., Gong, X., Cai, Y., Zhang, C., Yu, X., Fan, J., Diao, G.: Investigation of water-soluble inclusion complex of hypericin with β-cyclodextrin polymer, Carbohydr. Polym. 95, 366–370 (2013)
Wintgens, V., Amiel, C.: Water-soluble γ-cyclodextrin polymers with high molecular weight and their complex forming properties. Eur. Polym. J. 46, 1915–1922 (2010)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Patro, N.M., Sultana, A., Terao, K., Nakata, D., Jo, A., Urano, A., Ishida, Y., Gorantla, R.N., Pandit, V., Devi, K., Rohit, S., Grewal, B.K., Sophia, E.M., Suresh, A., Ekbote, V.K., Suresh, S.: Comparison and correlation of in vitro, in vivo and in silico evaluations of alpha, beta and gamma cyclodextrin complexes of curcumin. J. Incl. Phenom. Macrocycl. Chem. 78, 471–483 (2014)
Fenyvesi, É., Otta, K., Kolbe, I., Novák, C., Szejtli, J.: Cyclodextrin Complexes of UV Filters. J. Incl. Phenom. Macrocycl. Chem. 48, 117–123 (2004)
Tønnesen, H.H., Másson, M., Loftsson, T.: Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 244, 127–135 (2002)
Yamaji, M., Kida, M.: Photothermal tautomerization of a UV sunscreen (4-tert-butyl-4′-methoxydibenzoylmethane) in acetonitrile studied by steady-state and laser flash photolysis. J. Phys. Chem. A 117, 1946–1951 (2013)
Acknowledgments
The authors acknowledged the financial support from Kasetsart University Research and Development Institute (KURDI-10.57) and the Faculty of Science, Kasetsart University (URMF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karpkird, T., Khunsakorn, R., Noptheeranuphap, C. et al. Photostability of water-soluble inclusion complexes of UV-filters and curcumin with gamma-cyclodextrin polymer. J Incl Phenom Macrocycl Chem 84, 121–128 (2016). https://doi.org/10.1007/s10847-015-0589-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0589-5