Abstract
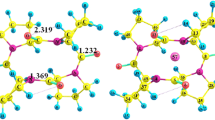
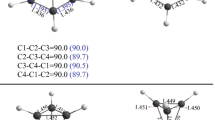
We studied the complexation of cyclo(L-Pro)3 with alkali metal cations (Li+, Na+, K+, Rb+ and Cs+) in the gas phase using density functional theory (DFT) calculations. The complexes were optimized at B3LYP/6-31+G(d) and CAM-B3LYP/6-31+G(d) levels of theory. The binding energy of M+-cyclo(L-Pro)3 complexes was increased in the following order: Li+ > Na+ > K+ > Rb+ > Cs+. Natural bond orbital (NBO) analysis at B3LYP/6-31+G (d) level was performed on the optimized geometries. These results indicated that the complexation in M+-cyclo(L-Pro)3 complexes, was caused by the lone pair electrons of electron donating oxygen atoms and the LP* orbitals of alkali cations. The electron density at bond critical points was consistent with the binding energy of M+-cyclo(L-Pro)3 complexes.




Similar content being viewed by others
References
Chen, G.J., Su, S., Liu, R.Z.: Theoretical studies of monomer and dimer of cyclo[(-1-phe1d-ala2-)n] and cyclo [(-1-phe1-d-MeN-Ala2-)n] (n = 3-6). J. Phys. Chem. B. 106, 1570–1575 (2002)
Teranishi, M., Okamoto, H., Takeda, K., Nomura, K., Nakano, A., Kalia, R.K.: Vashishta, Shimojo, P. F.: Molecular dynamic approach to the conformational transition in peptide nanorings and nanotubes. J. Phys. Chem. B. 113, 1473–1484 (2009)
Hartgerink, J.D., Granja, J.R., Milligan, R.A., Ghadiri, M.R.: Self-assembling peptide nanotubes. J. Am. Chem. Soc. 118, 43–50 (1996)
Tan, H.W., Qu, W.W., Chen, G.J., Liu, R.Z.: Theoretical investigation of the self-assembly of cyclo[(-β3-HGly)4-]. Chem. Phys. Lett. 369, 556–562 (2003)
Poteau, R., Trinquier, G.: All-cis cyclic peptides. J. Am. Chem. Soc. 127, 13875–13889 (2005)
Boyle, T.P., Bremner, J.P., Coates, J., Deadman, J., Keller, P.A.: New cyclic peptides via ring-closing metathesis reactions and their anti-bacterial activities. Tetrahedron 64, 11270–11290 (2008)
Cheng, L., Naumann, T.A., Horswill, A.R., Hong, S.-J., Venters, B.J., Tomsho, J.W., Benkovic, S.J., Keiler, K.C.: Discovery of antibacterial cyclic peptides that inhibit the ClpXP protease. Protein Sci. 16, 1535–1542 (2007)
Femandez-Lopez, S., Kim, H.-S., Choi, B.C., Delgado, M., Granja, J.R., Khasanov, A., Kraehenbuehl, K., Long, G., Weinberger, D.A., Wilcoxen, K.M., Ghadiri, M.R.: Antibacterial agents based on the cyclic D, L-a-peptide architecture. Nature 412, 452–455 (2001)
Matsumoto, T., Nishimura, K., Takeya, K.: New Cyclic Peptides from Citrus medica var. sarcodactylis SWINGLE. Chem. Pharm. Bull. 50, 857–860 (2002)
Maryanoff, B.E., Greco, M.N., Zhang, H.-C., Andrade-Gordon, P., Kauffman, J.A., Nicolaou, K.C., Liu, A., Brungs, P.H.: Macrocyclic Peptide inhibitors of serine proteases. Convergent total synthesis of cyclotheonamides A and B via a late-stage primary amine intermediate study of thrombin inhibition under diverse conditions. J. Am. Chem. Soc. 117, 1225–1239 (1995)
Dahiya, R.: Synthesis of a phenylalanine-rich peptide as potential anthelmintic and cytotoxic agent. Acta Polon. Pharm.-Drug Res. 64, 509–516 (2007)
Brasun, J., Witkiewicz, A.M., Ołdziej, S., Pratesi, A., Ginanneschi, M., Messori, L.: Impact of ring size on the copper(II) coordination abilities of cyclic peptides. J. Inorg. Biochem. 103, 813–817 (2009)
Ross, A.R.S., Luettgen, S.L.: Speciation of Cyclo(Pro-Gly)3 and its divalent metal-ion complexes by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 16, 1536–1544 (2005)
Praveena, G., Kolandaivel, P.: Interaction of metal ions with Cyclo [(1R,3S)-γ-Acc-Gly]3 hexapeptide-A theoretical study. J. Mol. Struct. (Theochem) 900, 96–102 (2009)
Montenegro, J., Ghadiri, M.R., Granja, J.R.: Ion channel models based on self-assembling cyclic peptide nanotubes. Acc. Chem. Res. 46, 2955–2965 (2013)
Sansom, M.S.P.: Alamethicin and related peptaibols-model ion channels. Eur. Biophys. J. 22, 105–124 (1993)
Ghadiri, M.R., Granja, J.R., Buehler, L.K.: Artificial transmembrane ion channels from self assembling peptide nanotubes. Nature 369(6478), 301–304 (1994)
Wang, D., Guo, L., Zhang, J., Roeske, R.W., Jones, L.R., Chen, Z., Pritchard, C.: Artificial ion channels formed by a synthetic cyclic peptide. J. Peptide. Res. 57, 301–306 (2001)
Chapman, R., Danial, M., Koh, M.L., Jolliffe, K.A., Perrier, S.: Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem. Soc. Rev. 41, 6023–6041 (2012)
Kubik, S.: Large increase in cation binding affinity of artificial cyclopeptide receptoresby an allosteric effect. J. Am. Chem. Soc. 121, 5846–5855 (1999)
Hioki, H., Kinami, H., Yoshida, A., Kojima, A., Kodama, M., Takaoka, S., Uedab, K., Katsu, T.: Synthesis of N-substituted cyclic triglycines and their response to metal ions. Tetrahedron Lett. 45, 1091–1094 (2004)
Izzo, I., Ianniello, G., Cola, C.D., Nardone, B., Erra, L., Vaughan, G., Tedesco, C., Riccardis, F.D.: Structural effects of proline substitution and metal binding on hexameric cyclic peptoides. Org. Lett. 15, 598–601 (2013)
Joshi, K.B., Verma, S.: Monovalent cation-promoted ordering of a glycine-rich cyclic peptides. Tetrahedron 63, 5602–5607 (2007)
Benedetti, E., Bavoso, A.: A., Blasio, B. D., Pavone, V., Pedone, C., Rossl, F.: Cyclic peptide metal salt adduct. II. Crystal structure of the silver nitrate cyclosarcosylsarcosine 2:1 adduct. Inorg. Chim. Acta 116, 31–35 (1986)
Brasun, J., Matera, A., Ołdziej, S., Kozłowska, J., Messor, L., Gabbiani, C.: Orfei, Ginanneschi, M.: The copper (II) coordination abilities of three novel cyclic tetrapeptides with –his-Xaa-His- motif. J. Inorg. Biochem. 101, 452–460 (2007)
Madison, V., Deber, C.M., Blout, E.R.: Cyclic peptides. 17. Metal and amino acid complexes of cyclo(Pro-Gly)4 and analogues studied by nuclear magnetic resonance and circular dichroism. J. Am. Chem. Soc. 99, 4788–4798 (1977)
Praveena, G., Kolandaivel, P.: Metal ion binding of α-γ hybrid cyclic peptid nanotubes-A theoretical study based on the ONIOM method. The IEEE Transactionson Nanobioscience. 9, 100–110 (2010)
Chermahini, A.N., Rezapour, M., Teimouri, A.: Selective complexation of alkali metal ions and nanotubular cyclopeptides: a DFT study. J. Incl. Phenom. Macrocycl. Chem. 79, 205–2014 (2014)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 37, 785–789 (1988)
Becke, A.: D.: density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Kamiya, M., Tsuneda, T., Hirao, K.: A density functional study of van der waals interactions. J. Chem. Phys. 117(13), 6010–6015 (2002)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange-correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393(1–3), 51–57 (2004)
van Duijneveldt, F.B., Van Duijneveldt-van de Rijdt, J.G., Jeanne G.C.M., Van Lenthe, J.H.: State of the art in counterpoise theory. Chem. Rev. 94, 1873–1885 (1994)
Boys, S.F., Bernardi, F.: The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970)
Reed, A.E., Weinstock, R.B.: Natural population analysis. J. Chem. Phys. 83, 735–746 (1985)
Bader, R.F.W.: Atoms in molecules, a quantum theory. International Series of Monographs in Chemistry. Oxford University Press, Oxford (1990)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, M.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J., Gaussian 09, Revision A.01, Gaussian Inc., Wallingford, (2009)
Druyan, M.E., Coulter, C.L., Walter, R., Kartha, G., Arnbady, G.K.: Structure and conformation of cyclo(tri-L-Prolyl) in the crystalline state. J. Am. Chem. Soc. 98, 5496–5502 (1976)
De Leeuw, F.A.A.M., Altona, C., Kessler, H., Bermel, W., Friedrich, A., Krack, G., Hul, W.: E: Conformational analysis of proline rings from proton spin-spin coupling constants and force-field calculations: Application to three cyclic tripeptides. J. Am. Chem. Soc. 105, 2237–2246 (1983)
Lowe, S.L., Pandey, R.R., Czlapinski, J., Kie-Adams, G., Hoffmann, M.R., Thomasson, K.A., Pierce, K.S.: Dipole interaction model predicted π − π* circular dichroism of cyclo(l-Pro)3 using structures created by semi-empirical, ab initio, and molecular mechanics methods. J. Peptide Res. 61, 189–201 (2003)
Diao, K.-S., Wang, H.-J., Qiu, Z.-M.: A DFT study on the selective extraction of metallic ions by 12-crown-4. J. Solution Chem. 38, 713–724 (2009)
Singh, R.N., Kumar, A., Tiwari, R.K., Rawat, P.: A combined experimental and theoretical (DFT and AIM) studies on synthesis, molecular structure, spectroscopic properties and multiple interactions analysis in a novel Ethyl-4-[2-(thiocarbamoyl)hydrazinylidene]-3,5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. Spectrochim. Acta A. 112, 182–190 (2013)
Bader, R.F.W.: A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991)
Popelier, P.L.A.: On the full topology of the Laplacian of the electron density. Coord. Chem. Rev. 197, 169–189 (2000)
Oliveira, B.G.: A DFT and AIM study of blue-shifting hydrogen bonds and secondary interactions in small heterocyclic complexes. J. Argent J. Chem. Soc. 95, 59–69 (2007)
Acknowledgments
We would like to acknowledge the Isfahan University of Technology for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chermahini, Z.J., Chermahini, A.N., Dabbagh, H.A. et al. Complexation of all-cis cyclo(L-Pro)3 and alkali metal cations: a DFT study. J Incl Phenom Macrocycl Chem 81, 465–473 (2015). https://doi.org/10.1007/s10847-015-0476-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0476-0