Abstract
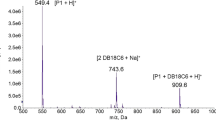
The ability of the crown ethers (1–4), containing the ortho- or para- methoxyphenoxy-methyl substituents in their structure, to chiral recognition in reference to amino acid esters has been investigated by electrospray ionization mass spectrometry (ESI-MS). The method allows registering the diastereomeric complexes between the studied crowns as hosts and the protonated alanine, phenylglycine and phenylalanine methyl esters as guests in the gas phase. ESI-MS experiments using isotopically labeled guests provide robust and reproducible results, indicating a moderate degree of chiral discrimination in the series of the studied crown ethers. ESI-MS experiments using achiral amine as a reference yielded the results comparable with the previous method. It has been found that (S)-enantiomers of the crowns bind predominately (S)-enantiomers of the amino acid esters, and vice-versa. It has been shown that the chiral ortho-substituted crown (S)-1 demonstrates the more pronounced values for chiral discrimination as compared with the para-substituted crown (S)-2. This fact indicates the interrelationship between the chiral recognition and the lariat nature of crown 1. Increasing the size of the cavity and the presence of a flat aromatic moiety in crowns 3 and 4 strengthens their complexing ability, simultaneously weakening the enantioselectivity of the complexation.








Similar content being viewed by others
References
Zhang, M., Zhu, K., Huang, F.: Improved complexation of paraquat derivatives by the formation of crown ether-based cryptands. Chem. Commun. 46, 8131–8141 (2010)
Tsukanov, A.V., Dubonosov, A.D., Bren, V.A., Minkin, V.I.: Organic chemosensors with crown-ether fragments. Chem. Heterocycl. Compd. 44, 899–921 (2008)
Gokel, G.W., Leevy, W.M., Weber, M.E.: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 104, 2723–2750 (2004)
Spath, A., Knig, B.: Molecular recognition of organic ammonium ions in solution using synthetic receptors. Beilstein J. Org. Chem. 6, 1–111 (2010)
Zhang, X.X., Bradshaw, J.S., Izatt, R.M.: Enantiomeric recognition of amine compounds by chiral macrocyclic receptors. Chem. Rev. 95, 3313–3362 (1997)
Gokel, G.W., Schall, O.F.: Lariat ethers. In: Gokel, G.W. (ed.) Comprehensive Supramolecular Chemistry, pp. 97–152. Pergamon, New York (1996)
Abbas, A.A., Elwahy, A.H.M.: Synthesis of C-pivot lariat ethers. J. Heterocycl. Chem. 46, 1035–1079 (2009)
Hyun, M.H.: Characterization of liquid chromatographic chiral separation on chiral crown ether stationary phases. J. Sep. Sci. 26, 242–250 (2003)
Lämmerhofer, M.: Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J. Chromatogr. A 1217, 814–856 (2010)
Paik, M.-J., Kang, J.S., Huang, B.S., Carey, J.R., Lee, W.: Development and application of chiral crown ethers as selectors for chiral separation in high-performance liquid chromatography and nuclear magnetic resonance spectroscopy. J. Chromatogr. A 1274, 1–5 (2013)
Hembury, G.A., Borovkov, V.V., Inoue, Y.: Chirality-sensing supramolecular systems. Chem. Rev. 108, 1–73 (2008)
Nakatsuji, Y., Nakahara, Y., Muramatsu, A., Kida, T., Akashi, M.: Novel C2-symmetric chiral 18-crown-6 derivatives with two aromatic sidearms as chiral NMR discriminating agents. Tetrahedron Lett. 46, 4331–4335 (2005)
Ema, T.: Synthetic macrocyclic receptors in chiral analysis and separation. J. Incl. Phenom. Macrocycl. Chem. 74, 41–55 (2012)
Zhou, L., Lin, Z., Reamer, R.A., Mao, B., Ge, Z.: Stereoisomeric separation of pharmaceutical compounds using CE with a chiral crown ether. Electrophoresis 28, 2658–2666 (2007)
Schug, K.A., Lindner, W.: Chiral molecular recognition for the detection and analysis of enantiomers by mass spectrometric methods. J. Sep. Sci. 28, 1932–1955 (2005)
Speranza, M.: Enantioselectivity in gas-phase ion-molecule reactions. Int. J. Mass Spectrom. 232, 277–317 (2004)
Sawada, M., Takai, Y., Yamada, H., Kaneda, T., Kamada, K., Mizooku, T., Hirose, K., Tobe, Y., Naemura, K.: Chiral amino acid recognition detected by electrospray ionization (ESI) and fast atom bombardment (FAB) mass spectrometry (MS) coupled with the enantiomer-labeled (EL) guest method. J. Chem. Soc. Perkins Trans. II 3, 701–710 (1998)
Liang, Y., Bradshaw, J.S., Izatt, R.M., Pope, R.M., Dearden, D.V.: Analysis of enantiomeric excess using mass spectrometry: fast atom bombardment/sector and electrospray ionization/Fourier transform mass spectrometric approaches. Int. J. Mass Spectrom. 185/186/187, 977–988 (1999)
Gerbaux, P., De Winter, J., Cornil, D., Ravicini, K., Pesesse, G., Cornil, J., Flammang, R.: Noncovalent interactions between ([18]crown-6)-tetracarboxylic acid and amino acids: electrospray-ionization mass spectrometry investigation of the chiral-recognition processes. Chem. Eur. J. 14, 11039–11049 (2008)
Sawada, M., Takai, Y., Yamada, H., Yoshikawa, M., Arakawa, R., Tabuchi, H., Takada, M., Tanaka, J.: Depression of the apparent chiral recognition ability obtained in the host-guest complexation systems by electrospray and nano-electrospray ionization mass spectrometry. Eur. J. Mass Spectrom. 10, 27–37 (2004)
Schalley, Ch.A.: Molecular recognition and supramolecular chemistry. Mass Spectrom. Rev. 20, 253–309 (2001)
Cera, L., Schalley, Ch.A.: Supramolecular reactivity in the gas phase: investigating the intrinsic properties of non-covalent complexes. Chem. Soc. Rev. 43, 1800–1812 (2014)
Sawada, M., Takai, Y., Yamada, H., Hirayama, S., Kaneda, T., Tanaka, T., Kamada, K., Mizooku, T., Takeuchi, S., Ueno, K., Hirose, K., Tobe, Y., Naemura, K.: Chiral recognition in host-guest complexation determined by the enantiomer-labeled guest method using fast atom bombardment mass spectrometry. J. Am. Chem. Soc. 117, 7726–7736 (1995)
Steed, J.W., Atwood, J.L.: Supramolecular Chemistry, 2nd edn. Wiley, Chichester (2009)
Sharafutdinova, D.R., Fayzullin, R.R., Bazanova, O.V., Bredikhina, Z.A., Rizvanov, I.H., Bredikhin, A.A.: Mass spectrometric investigation of the side-arm lariat effect of ortho- and para-methoxyphenoxymethyl-15-crown-5 in the gas phase. J. Anal. Chem. 68, 1178–1182 (2013)
Bredikhin, A.A., Gubaidullin, A.T., Bredikhina, Z.A., Fayzullin, R.R.: Crystallographic evidence of side-arm lariat effect in the series of chiral ortho- and para-methoxyphenoxymethyl-15-crown-5 complexes with sodium perchlorate. J. Mol. Struct. 1032, 176–184 (2013)
Bredikhina, Z.A., Eliseenkova, R.M., Fayzullin, R.R., Novikova, V.G., Kharlamov, S.V., Sharafutdinova, D.R., Latypov, Sh.K, Bredikhin, A.A.: Synthesis and extraction properties of some lariat ethers derived from the spontaneously resolved guaifenesin, 3-(2-methoxyphenoxy)propane-1,2-diol. ARKIVOC (x) 16–32 (2011)
Bredikhina, Z.A., Novikova, V.G., Zakharychev, D.V., Bredikhin, A.A.: Solid state properties and effective resolution procedure for guaifenesin, 3-(2-metoxyphenoxy)-1,2-propanediol. Tetrahedron Asymmetry 17, 3015–3020 (2006)
Kristensen, T.E., Vestli, K., Hansen, F.K., Hansen, T.: New phenylglycine-derived primary amine organocatalysts for the preparation of optically active warfarin. Eur. J. Org. Chem. 30, 5185–5191 (2009)
Kyba, E.P., Timko, J.M., Kaplan, L.J., de Jong, F., Gokel, G.W., Cram, D.J.: Host-guest complexation. 11. Survey of chiral recognition of amine and amino ester salts by dilocular bisdinaphthyl hosts. J. Am. Chem. Soc. 100, 4555–4568 (1978)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bredikhina, Z.A., Sharafutdinova, D.R., Bazanova, O.B. et al. Lariat ethers in the chiral recognition of amino acid esters:electrospray ionization mass spectrometry investigation. J Incl Phenom Macrocycl Chem 80, 417–426 (2014). https://doi.org/10.1007/s10847-014-0430-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0430-6