Abstract
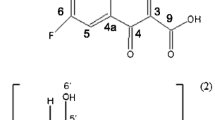
Host–guest interactions between the antifungal agent Octopirox® (Oc) and modified β-cyclodextrin-derivatives were studied using 1H- and 2D-ROESY NMR spectroscopy, Job-Plot and isothermal titration calorimetry (ITC). In addition to β-cyclodextrin (β-CD) a number of derivatives, namely randomly methacrylated β-cyclodextrin (RM-β-CD), mono-methacrylated β-cyclodextrin (MM-β-CD), randomly methylated β-cyclodextrin (RAMEB), hydroxypropyl-β-cyclodextrin (HP-β-CD) and randomly methacrylated hydroxypropyl-β-cyclodextrin (RM-HP-β-CD) were used. NMR data suggests the formation of highly ordered complexes, while ITC measurements allowed the identification of their stoichiometries and the thermodynamic data. To evaluate the possibility of retarded drug release from complexes prepared from polymeric materials like artificial nails, the complexes were polymerized with comonomers and subjected to aqueous extraction followed by quantification of Oc release by means of UV-spectroscopy.








Similar content being viewed by others
References
Futterer, E.: Antidandruff hair tonic containing piroctone olamine. Cosmet. Toilet. 103, 49–52 (1988)
Dietrich, G., Bollert, V.: Praxisnahe Prüfmethode für Wirkstoffe gegen vermehrte Schuppung der Kopfhaut. Ärztliche Kosmetologie 10, 34–45 (1980)
Futterer, E.: Evaluation of efficacy of antidandruff agents. J. Soc. Cosmet. Chem. 32, 327–338 (1981)
Watanabe, Y., Yokoyama, M., Yamada, K., Arima, M., Hori, T., Sagai, M.: Clinical Evaluation of Hair Shampoo and Hair Rinse Containing Piroctone Olamine. J. Jpn. Soc. Cosmet. Sci. 6(2), 79–99 (1982)
Futterer, E.: Untersuchung zur Wirksamkeit löslicher Antischuppenwirkstoffe. Ärztliche Kosmetologie 15, 421–435 (1985)
Black, J.G., Kamat, V.B.: Percutaneous absorption of octopirox. Food Chem. Toxicol. 26, 53–58 (1988)
Hashimoto, S., Uchino, N., Watari, Y.: Technological progress in formulation and manufacture of medicated shampoo. Fragr J. Special Issue 7, 62–67 (1986)
Schrader, K.: Comparative experimental research on dandruff through quantitative image analysis. J. Appl. Cosmetol. 4, 153–170 (1986)
Schrader, K., Bielefeldt, S.: Vergleichende experimentelle Untersuchungen von Kopfschuppen mit der quantitativen Bildanalyse. Parfümerie und Kosmetik 68, 72–80 (1987)
Myfungar® Nail polish; Polichem SA, Lugano (Switzerland); distributed by Taurus Pharma GmbH, Frankfurt/Main (Germany)
Dubini, F., Belotti, M.G., Frangi, A., Monit, D., Saccomani, L.: In vitro antimycotic activity and nail permeation models of a piroctone olamine (octopirox) containing transungual water soluble technology. Arzneim.-Forsch./Drug Res. 55(8), 478–483 (2005)
Gröger, M., et. Al.: Cyclodextrine. http://www.science-forum.de/download/cyclodex.pdf. Accessed 1 Oct 2012
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Ritter, H., Tabatabei, M.: Grüne Polymerchemie—polymerisationsverfahren in Wasser unter Verwendung von Cyclodextrinen. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-809/pagesritter.pdf. Accessed 1 Oct 2012
Forrest, M.L., Gabrielson, N., Pack, D.W.: Cyclodextrin–polyethylenimine conjugates for targeted in vitro gene delivery. Biotechnol. Bioeng. 89, 416–423 (2005)
Kretschmann, O., Choi, S.W., Miyauchi, M., Tomatsu, I., Harada, A., Ritter, H.: Switchable hydrogels obtained by supramolecular cross-linking of adamantyl-containing LCST copolymers with cyclodextrin dimers. Angew. Chem. Int. Ed 45, 4361–4365 (2006)
Kretschmann, O., Ritter, H.: Copolymerization of fluorinated monomers with hydrophilic monomers in aqueous solution in presence of cyclodextrin. Macromol. Chem. Phys. 207, 987–992 (2006)
Antonietti, L., Aymonier, C., Schlotterbeck, U., Garamus, V.M., Maksimova, T., Richtering, W., Mecking, S.: Core-shell-structured highly branched poly(ethylenimine amide)s: synthesis and structure. Macromolecules 38, 5914–5920 (2005)
Maciollek, A., Munteanu, M., Ritter, H.: New generation of polymeric drugs: copolymer from NIPAAM and cyclodextrin methacrylate containing supramolecular-attached antitumor derivative. Macromol. Chem. Phys. 211, 245–249 (2010)
Zhou, J., Ritter, H.: Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 1, 1552–1559 (2010)
Valentino, J.S., Quanren, H.: Cyclodextrins. Toxicol. Pathol. 36, 30–42 (2008)
Dos Santos, J.-F.R., Couceiro, R., Concheiro, A., Torres-Labandeira, J–.J., Alvarez-Lorenzo, A.: Poly(hydroxyethyl methacrylate-co-methacrylated-b-cyclodextrin) hydrogels: synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomater. 4, 745–755 (2008)
Uekama, K.: Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 52, 900–915 (2004)
Hoare, T.R., Kohane, D.S.: Hydrogels in drug delivery: progress and challenges. Polymer 49, 1993–2007 (2008)
Bouchemal, K.: Drug Discov Today 13, 960–972 (2008)
Denadai, A.M.L., Santoro, M.M., Da Silva, L.H., Viana, A.T., Dos Santos, R.A.S., Sinisterra, R.D.J.: Self-assembly Characterization of the β-cyclodextrin and hydrochlorothiazide system: NMR, phase solubility, ITC and QELS. Inclusion Phenom. Macrocyl. Chem. 55, 41–49 (2006)
Teixeira, L.R., Sinisterra, R.D., Vieira, R.P., Scarlatelli-Lima, A., Moraes, M.F.D., Doretto, M.C., Denadai, A.M., Beraldo, H.: An Inclusion compound of the anticonvulsant sodium valproate into a-cyclodextrin: physico-chemical haracterization. J. Inclusion Phenom. Macrocycl. Chem. 54, 133–138 (2006)
Schneider, H.J., Hacket, F., Rudiger, V., Ikeda, H.: NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98, 1755–1785 (1998)
Rahman, A.: One and two dimensional NMR spectroscopy, 1st edn. Elsevier, New York (1989)
Ohga, K., Takashima, Y., Takashima, H., Kawaguchi, Y., Yamaguchi, H., Harada, A.: Preparation of supramolecular polymers from a cyclodextrin dimer and ditopic guest molecules: control of structure by linker flexibility. Macromolecules 38, 5897–5904 (2005)
Kretschmann, O.: Diss., Assoziative Hydrogele und thermosensitive Polymer-Einschlussverbindungen auf Basis von adamantylhaltigen Polymeren und Cyclodextrinen, 95–98 (2006)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Thompson, D.O.: Cyclodextrins–enabling excipients: their present and future use in pharmaceuticals. Crit. Rev. Ther. Drug Carrier Syst. 14, 1–104 (1997)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
Rekharsky, M.V., Yamamura, H., Kawai, M., Inoue, Y.: Complexation and chiral recognition thermodynamics of gamma-cyclodextrin with N-acetyl- and N-carbobenzyloxy-dipeptides possessing two aromatic rings. J. Org. Chem. 68, 5228–5235 (2003)
Praefcke, G.J.K.: Isotherme Titrationskalorimetrie (ITC) zur Charakterisierung biomolekularer Wechselwirkungen. BIOspektrum 1, 44–47 (2005)
Turnbull, W.B., Daranas, A.H.: On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J. Am. Chem. Soc. 125, 14859–14866 (2003)
Harrison, J.C.: Cyclodextrin–adamantanecarboxylate inclusion complexes: a model system for the hydrophobic effect. Biopolymers 21, 1153–1166 (1982)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dollendorf, C., Maier, M., Janda, R. et al. Study of the interaction between modified cyclodextrin and octopriox : potential applications in drug delivery. J Incl Phenom Macrocycl Chem 77, 351–361 (2013). https://doi.org/10.1007/s10847-012-0254-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0254-1