Abstract
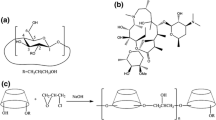
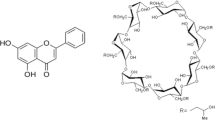
The aim of this study was to investigate the effect of hydroxypropyl-β-cyclodextrin (HPβCD) on the solubility and dissolution rate of Cefdinir (CEF). The methods that were employed to prepare CEF–HPβCD complexes were Kneading (KN), Co-evaporation (CE), Spray drying (SD) and a novel approach of Microwave irradiation (MWI). The formation of inclusion complexes with HPβCD in the solid state, were characterized by Differential Scanning Calorimetry (DSC), Fourier Transformation Infrared spectroscopy (FTIR), Proton Nuclear Magnetic Resonance Spectroscopy (NMR), X Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) studies, and comparative studies on the in vitro dissolution of CEF were carried out. Phase solubility profile with HPβCD was classified as AL type, indicating the formation of 1:1 stoichiometric inclusion complexes. Characterization of binary systems by DSC, FTIR, NMR, XRD and SEM indicated that SD and MWI method resulted in formation of true complexes. Binary systems showed significant increase in dissolution rate as compared to plain drug. Amongst the various binary systems, MWI products were prepared in least time with better yield and highest dissolution rate.








Similar content being viewed by others
References
Cabri, W., Ghetti, P., Alpegiani, M., Pozzi, Giovanni., Justo-Erbez, A., Perez-Martinez, J.I., Villalon-Rubio, R., Monedero-Perales, M.C., Munoz-Rubio, A.: Cefdinir: a comparative e study of anhydrous vs monohydrate form Microstructure and tableting behaviour. Eur. J. Pharm. Biopharm 64, 212–221 (2006). doi:10.1016/j.ejpb.2006.05.007
Inamoto, Y., Chiba, T., Kamimura, T., Takaya, T.: FK 482, a new orally active cephalosporin synthesis and biological properties. J. Antibiot. 41, 828–830 (1988)
Szejtli, J.: Cyclodextrin Technology, pp. 1–78. Kluwer Academic Publishers, Dordrecht (1988)
Duchene, D., Wouessidjewe, D.: Pharmaceutical uses of cyclodextrins and derivatives. Drug Dev. Ind. Pharm. 16(17), 2487–2499 (1990)
Loftsson, T., Bjornsdottir, S., Palsdottir, G., Bodor, N.: Cyclodextrins. The effects of 2-hydroxypropyl-β-cyclodextrin on the solubility and stability of chlorambucil and melphalan in a aqueous solution. Int. J. Pharm 57, 63–72 (1989)
Bekers, O., Beijnen, J.M., Kempers, Y.A.G., Bult, A., Underberg, J.M.: Eeeects of cyclodextrins on N-trifluoroacetyl-doxorubicin-14-valerate (AD-32) stability and solubility in aqueous media. Int. J. Pharm 68, 271–276 (1991)
Vila-Jato, J.L., Blanco, J., Torres, J.J.: Biopharmaceutical aspects of the tolbutamide-β-cyclodextrin inclusion compound. II Farmaco Edizione Pratica 643(2), 37–45 (1988)
Le Bas, D., Rysanek, N.: Structural aspects of cyclodextrins. In: Duchene, D. (ed.) Cyclodextrins and Their Industrial Uses, pp. 105–125. Editions de SAnte, Paris (1987)
Loftsson, T., Brewster, M.E., Derendorf, H., Bodor, N.: 2-Hydroxypropyl-β-cyclodextrin. Properties and usage in pharmaceutical formulations. Pharm. Ztg. Wiss. 136(1–4), 5–10 (1991)
Esclusa-Diaz, M.T., Torres-Labandeira, J.J., Kata, M., Vila-Jato, J.L.: Inclusion complexation of glibenclamide with 2-hydroxypropyl- β-cyclodextrin in solution and in solid state. Eur. J. Pharm. Biopharm. 1, 291–296 (1994)
Ribeiro, L., Carvalho, R.A., Ferreira, D.C., Veiga, F.J.B.: Multicomponent complex formation between vinpocetine, cyclodextrins, tartaric acid and water soluble polymers monitored by NMR and solubility studies. Eur. J. Pharm. Sci. 24, 1–13 (2005)
Parmar, K.R., Patel, K.A., Shah, S.R., Sheth, N.R.: Inclusion complexes of lamotrigine and hydroxypropyl-β-cyclodextrin: solid state characterization and dissolution studies. J. Incl. Phenom. Macrocycl. Chem. 65, 263–268 (2009)
Szente, L., Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Vogtle, F.: Comprehensive Supramolecular Chemistry, vol. 3, p. 243. Elsevier Science Ltd., Oxford (1996)
Zhao, D.Y., Yang, S.G., Hu, M., Ma, X.Y.: Structural study of inclusion complex of andrographolide with β-cyclodextrin prepared under microwave irradiation. Chin. Chem. Lett. 14, 155–158 (2003)
Zhao, D., Liao, K., Ma, X., Yan, N.: Study of supramolecular inclusion of β-cyclodextrin with andrographolide. J. Incl. Phenom. Macrocycl. Chem. 43, 259–264 (2002)
Kappe, C.O.: Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 43, 6250–6284 (2004)
Zhou, J., Shi, C., Mei, B., Yuan, R., Fu, Z.: Research on the technology and the mechanical properties of the microwave processing of polymer. J. Mater. Process. Tech. 137, 156–158 (2003)
Passerini, N., Albertini, B., González-Rodríguez, M.L., Cavallari, C., Rodriguez, L.: Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur. J. Pharm. Biopharm. 15, 71–78 (2002)
Moneghini, M., Bellich, B., Baxa, P., Princivalle, F.: Microwave generated solid dispersions containing Ibufrofen. Int. J. Pharm. 361, 125–130 (2008)
Wen, X., Tan, F., Jing, Z., Liu, Z.: Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharm. Biomed. Anal. 34(3), 517–523 (2004)
Aleem, O., Kuchekar, B., Pore, Y., Late, S.: Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of Cefdinir. J. Pharm. Biomed. Anal. 47, 535–540 (2008). doi:10.1016/jpba.2008.02.006
Ren, Y., Gao, J., Han, B., Ma, X.: Medical composition containing cyclodextrin inclusion compound of cefdinir and its preparation method. PCT Int. Appl. PCT/CN2008/000514 (2008)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Bodmeier, R., Chen, H.: Preparation of biodegradable poly (±) lactide microparticles using a spray-drying technique. J. Pharm. Pharma¢ol 40, 754–757 (1988)
Benesi, H., Hildebrand, J.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbon. J. Am. Chem. Soc. 71, 2701–2705 (1949)
Spulber, M., Pinteala, M., Harbagiu, V., Simionescu, B.C.: Inclusion complexes of Sulconazole with b-cyclodextrin and hydroxypropyl β-cyclodextrin: characterization in aqueous solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 61, 41–51 (2008)
Esclusa-Diaz, M.T., Guimaraens-Mfndez, M., Pfrez-Marcos, M.B., Vila-Jato, J.L., Torres-Labandeira, J.J.: Characterization and in vitro dissolution behaviour of ketoconazole/β- and 2-hydroxypropyl-β-cyclodextrin inclusion compounds. Int. J. Pharm. 143, 203–210 (1996)
Vij, M., Garse, H., Dand, N., Kadam, V., Hirlekar, R.: Effect of preparation method on complexation of Cefdinir with β-cyclodextrin. J. Inc. Phenom. Macrocycl. Chem. (2009). doi:10.1007/s10847-009-9666-y
Acknowledgment
The authors would like to place on record their sincere gratitude to Alkem Research Lab. Ltd (India) for kindly gifting Cefdinir and Wacker Fine Chemical Corporation (Germany) for generously providing HPβCD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohit, V., Harshal, G., Neha, D. et al. A comparative study of complexation methods for cefdinir-hydroxypropyl-β-cyclodextrin system. J Incl Phenom Macrocycl Chem 71, 57–66 (2011). https://doi.org/10.1007/s10847-010-9901-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9901-6