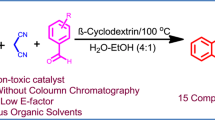
Abstract
An aqueous hydroxypropyl-β-cyclodextrin solution has been used to increase the conversion of 4-biphenylcarboxaldehyde into the corresponding alcoholic and carboxylic substrates, by means of a Cannizzaro reaction. The observed enhancement has been ascribed to a partial solubilization of 4-biphenylcarboxaldehyde. In addition, as the main part of the organic substrates still remains insoluble, synthesized products are easily recovered by filtration. As a consequence, the basic cyclodextrin solution might also be reused for a new synthetic cycle, without significant loss of conversion. Aqueous solid–liquid biphasic reaction in presence of cyclodextrins thus seems to be a promising tool in the green chemistry field.





Similar content being viewed by others
Abbreviations
- HPBCD:
-
Hydroxypropyl-β-cyclodextrin
References
Szejtli, J.: Chemistry, physical and biological properties of cyclodextrins. Compr. Supramol. Chem. 3, 5–40 (1996)
Del Valle, E.M.M.: Cylodextrins and their uses: a review. Process Biochem. 39(9), 1033–1046 (2004)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98(5), 2045–2076 (1998)
Dabbawala, A.A., Parmar, J.N., Jasra, R.V., Bajaj, H.C., Monflier, E.: Cobalt catalyzed hydroformylation of higher olefins in the presence of chemically modified cyclodextrins. Catal. Commun. 10(14), 1808–1812 (2009)
Hapiot, F., Leclercq, L., Azaroual, N., Fourmentin, S., Tilloy, S., Monflier, E.: Rhodium-catalyzed hydroformylation promoted by modified cyclodextrins: current scope and future developments. Curr. Org. Synth. 5(2), 162–172 (2008)
Bhosale, S.V., Bhosale, S.V.: β-Cyclodextrin as a catalyst in organic synthesis. Mini Rev. Org. Chem. 4(3), 231–242 (2007)
Kunishima, M., Watanabe, Y., Terao, K., Tani, S.: Substrate-specific amidation of carboxylic acids in a liquid–liquid two-phase system using cyclodextrins as inverse phase-transfer catalysts. Eur. J. Org. Chem. 22, 4535–4540 (2004)
Tilloy, S., Bricout, H., Monflier, E.: Cyclodextrins as inverse phase transfer catalysts for the biphasic catalytic hydrogenation of aldehydes: a green and easy alternative to conventional mass transfer promoters. Green Chem. 4(3), 188–193 (2002)
Kalck, P., Miquel, L., Dessoudeix, M.: Various approaches to transfers improvement during biphasic catalytic hydroformylation of heavy alkenes. Catal. Today 42(4), 431–440 (1998)
Bowden, K., El-Kaissi, F.A., Ranson, R.J.: Intramolecular catalysis. Part 5. The intramolecular Cannizzaro reaction of o-phthalaldehyde and [α, α′-2H2]-o-phthalaldehyde. J. Chem. Soc., Perkin Trans. 2 12, 2089–2092 (1990)
Basavaiah, D., Sharada, D.S., Veerendhar, A.: Organo-base mediated Cannizzaro reaction. Tetrahedron Lett. 47(32), 5771–5774 (2006)
Yang, X., Guo, J., Zou, G.: Lanthanide-catalysed cross-Cannizzaro reduction of aromatic aldehydes with formaldehyde. Lett. Org. Chem. 2(2), 145–147 (2005)
Bikbaeva, G.G., Kislina, I.S., Vinnik, M.I.: Kinetics of the cross Cannizzaro reaction of anisic aldehyde with formaldehyde in aqueous solutions of KOH. Bull. Acad. Sci. USSR CH+ 23(12), 2616–2620 (1975)
Abbaszadeh, M.R., Bowden, K.: Intramolecular catalysis. Part 4. The intramolecular Cannizzaro reaction of biphenyl-2,2-dicarbaldehyde, [α,α’-2H2]biphenyl-2,2′-dicarbaldehyde and 4,4′-or 5,5′- or 6,6′-disubstituted biphenyl-2,2′-dicarbaldehydes. J. Chem. Soc. Perkin Trans. 12(2), 2081–2087 (1990)
Bowden, K., Butt, A.M., Streater, M.: Intramolecular catalysis. Part 8. The intramolecular Cannizzaro reaction of naphthalene-1,8-dicarbaldehyde and [αα′-2H2]naphthalene-1,8-dicarbaldehyde. J. Chem. Soc., Perkin Trans. 2 4, 567–571 (1992)
Morooka, S., Wakai, C., Matubayasi, N., Nakahara, M.: Noncatalytic Cannizzaro-type reaction of acetaldehyde in supercritical water. Chem. Lett. 32, 310–311 (2003)
Sharifi, A., Mojtahedi, M.M., Saidi, M.R.: Microwave irradiation techniques for the Cannizzaro reaction. Tetrahedron Lett. 40(6), 1179–1180 (1999)
Mohamadi, F., Richards, N.G.J., Guida, W.C., Liskamp, R., Lipton, M., Caufield, C., Chang, G., Hendrickson, T., Still, W.C.: MacroModel—an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 11, 440–467 (1990)
Cheng, A., Best, S.A., Merz Jr, K.M., Reynolds, C.H.: GB/SA water model for the Merck molecular force field (MMFF). J. Mol. Graph. Model. 18(3), 273–282 (2000)
Acknowledgement
Authors wish to thank IRENI for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canipelle, M., Landy, D. & Fourmentin, S. Improved aqueous Cannizzaro reaction in presence of cyclodextrin. J Incl Phenom Macrocycl Chem 69, 349–353 (2011). https://doi.org/10.1007/s10847-010-9747-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9747-y