Abstract
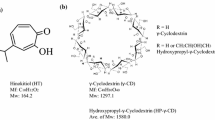
The method of co-grinding with cyclodextrins (CDs) was applied to a poorly water soluble drug, ONO-8713 (solubility; 0.92 μg/ml in H2O at 25°C) as a method to prepare nanoparticles. ONO−8713 was co−ground with various CDs in a vibration mill. α−Cyclodextrin, β−CD,$-CD, CD derivatives and some sugars were used as co-grinding additives. Suitable moisture content in the co-grinding system was required to achieve maximum nanoparticle yield. When ONO-8713 was co-ground with β-CD (molar ratio of β-CD:drug=5:1) at 12% moisture, 85% of drug recovered as nanoparticles with a mean particle size of 120 nm. Nanoparticle yield achieved 90% when hydroxypropyl-β-CD was used as a co-grinding additive. It was found that the amount of drug nanoparticles depended on the characteristics of CDs. This phenomenon was probably due to the difference in the cavity size of CDs along with the variation of substitution groups that affected the interaction between CDs and drug, and the affinity between CD and drug molecules. Zeta potential analysis suggested that CD would form a layer covering on the particle surface and alter the charge of the particles, improving the stability and total yield of the nanoparticle.
Similar content being viewed by others
References
MH El-Shabouri (2002) STP Pharma Sci 12 97
FJ Grcic D Voinovich M Moneghini MB Lacan L Magarotto I Jalsenjak (2000) Eur J Pharm Sci 9 373
D Kayrak U Akman O Hortaçsu (2003) J Supercrit Fluids 26 17
M Charoenchaitrakool F Dehghani N.R Foster HK Chan (2000) Ind Eng Chem Res 39 4794
G Nykamp U Carstensen BW Muller (2002) Int J Pharm 242 79
T Watanabe I Ohno N Wakiyama A Kusai M Senna (2002) Int J Pharm 241 103
Y Nakai K Yamamoto K Terada K Akimoto (1984) Chem Pharm Bull 32 685
T Oguchi Y Tozuka T Hanawa M. Mizutani N Sasaki S Limmatvapirat K Yamamoto (2002) Chem Pharm Bull 50 887
S Limmatvapirat E Yonemochi T Oguchi K Yamamoto (1997) Chem Pharm Bull 45 1358
H Kubo T Osawa K Takashima M Mizobe (1996) Chem Pharm Bull 19 741
M. Sugimoto T Okagaki S Narisawa Y Koida K Nakajima (1998) Int J Pharm 160 11
T Yamada N Saito T Imai M Otagiri (1999) Chem Pharm Bull 47 1311
A. Wongmekiat Y Tozuka T Oguchi K Yamamoto (2002) Pharm Res 19 1867
A Wongmekiat Y Tozuka T Oguchi K Yamamoto (2003) Int J Pharm 265 85
K Itoh A Pongpeerapat Y Tozuka T Oguchi K Yamamoto (2003) Chem Pharm Bull 51 171
R McCandless SH Yalkowsky (1998) J Pharm Sci 87 1639
G Dollo PL Corre M Chollet F Chevanne M Bertault JL Burgot RL Verge (1999) J Pharm Sci 88 889
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tozuka, Y., Wongmekiat, A., Sakata, K. et al. Co-grinding with Cyclodextrin as a Nanoparticle Preparation Method of a Poorly Water Soluble Drug. J Incl Phenom Macrocycl Chem 50, 67–71 (2004). https://doi.org/10.1007/s10847-003-8841-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10847-003-8841-9