Abstract
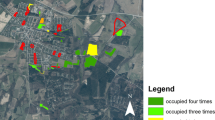
The Scarce Copper (Lycaena virgaureae) is a species that has suffered serious decline in several European countries. In Scandinavia it is still comparatively abundant, but with ongoing losses of flower-rich grasslands near forests further decline is expected. A mark–release–recapture study was carried out in July 2013 at 14 sites on the outskirts of a village located near Malmö, Sweden. The study area comprised in total an 11.4 ha network of abandoned agricultural sites, road verges and forest edges. A private garden was also included. Butterflies were marked individually and the capture position was recorded by GPS. Sex, behaviour and flower visits were also recorded. During the study 852 butterflies were marked and 170 of these were recaptured at least once (recapture rate 20 %), resulting in 1,078 captures (including multiple recaptures). Movement between patches accounted for 41 % of all recaptures and mean distance between recaptures was 112 ± 146 m (n = 226). The number of captures was strongly positively correlated with patch size (ρ = 0.95, p < 0.05), while the emigrant and immigrant fractions were significantly negatively correlated with patch size. Overall, the Scarce Copper was surprisingly abundant in the area, but planned construction of residential areas will result in the loss of most habitat patches.






Similar content being viewed by others
References
Baguette M, Clobert J, Schtickzelle N (2011) Metapopulation dynamics of the bog fritillary butterfly: experimental changes in habitat quality induced negative density-dependent dispersal. Ecography 34:170–176
Barua KK, Chuluunbaatar G, Muehlenberg M (2011) Population structure and mobility of the Scarce Copper Lycaena virgaureae in the herb meadow habitat of Northern Mongolia. J Entomol 8:40–51
Bergerot B, Fontaine B, Julliard R, Baguette M (2011) Landscape variables impact the structure and composition of butterfly assemblages along an urbanization gradient. Landsc Ecol 26:83–94
Bergerot B, Merckx T, Van Dyck H, Baguette M (2012) Habitat fragmentation impacts mobility in a common and widespread woodland butterfly: do sexes respond differently? BMC Ecol 12:5
Bergerot B, Tournant P, Moussus J-P, Stevens V-M, Julliard R, Baguette M, Foltete JC (2013) Coupling inter-patch movement models and landscape graph to assess functional connectivity. Popul Ecol 55:193–203
Bink FA (1992, cited in Schlumprecht and Bräu 2013) EcologischeAtlas van de Dagflinders van Noordwest-Europa. Schuyt, Harlem
Blair RB, Launer AE (1997) Butterfly diversity and human land use: species assemblages along an urban gradient. Biol Conserv 80:113–125
Bonelli S, Vrabec V, Witek M, Barbera F, Patricelli D, Nowicki P (2013) Selection on dispersal in isolated butterfly metapopulations. Popul Ecol 55:469–478
Breed GA, Stichter S, Crone EE (2013) Climate-driven changes in northeastern US butterfly communities. Nat Clim Chang 3:142–145
Clark PJ, Reed JM, Chew FS (2007) Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst 10:321–337
Dennis RLH, Hardy PB (2001) Loss rates of butterfly species with urban development. A test of atlas data and sampling artefacts at a fine scale. Biodivers Conserv 10:1831–1837
Di Mauro D, Dietz T, Rockwood L (2007) Determining the effect of urbanization on generalist butterfly species diversity in butterfly gardens. Urban Ecosyst 10:427–439
Douwes P (1975a) Territorial behavior in Heodes virgaureae L. (Lep. Lycaenidae) with particular reference to visual stimuli. Nor J Entomol 22:143–154
Douwes P (1975b) Distribution of a population of the butterfly Heodes virgaureae. Oikos 26:332–340
Dover JW, Spencer S, Collins S, Hadjigeorgiou I, Rescia A (2011) Grassland butterflies and low intensity farming in Europe. J Insect Conserv 15:129–137
Ebert G (ed) (1993) Die Schmetterlinge Baden-Württembergs, Tagfalter II, vol 2. Verlag, Eugen Ulmer, Stuttgart
ESRI (2012) ArcGIS Desktop: release 10.1. Environmental Systems Research Institute, Redlands, CA
Feest A, Van Swaay C, Van Hinsberg A (2014) Nitrogen deposition and the reduction of butterfly biodiversity quality in the Netherlands. Ecol Indic 39:115–119
Filz KJ, Engler JO, Stoffels J, Weitzel M, Schmitt T (2013) Missing the target? A critical view on butterfly conservation efforts on calcareous grasslands in south-western Germany. Biodivers Conserv 22:2223–2241
Fjellstad WJ (1998) The landscape ecology of butterflies in traditionally managed Norwegian farmland. Dissertation, University of Durham, UK
Henriksen HJ, Kreutzer I (1982) The butterflies of Scandinavia in nature. Skandinavisk Bogforlag, Odense
Hill JK, Thomas CD, Lewis OT (1996) Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J Anim Ecol 65:725–735
Inoue T (2005) Causes of butterfly decline in Japan. Jpn J Entomol New Ser 8:43–64
Kitahara M, Fujii K (1994) Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: an analysis based on the concept of generalist versus specialist strategies. Res Popul Ecol 36:187–199
Konvicka M, Kadlec T (2011) How to increase the value of urban areas for butterfly conservation? A lesson from Prague nature reserves and parks. Eur J Entomol 108:219–229
Kudrna O, Harpke A, Lux K, Pennerstorfer J, Schweiger O, Settele J, Wiemers M (2011) Distribution Atlas of Butterflies in Europe. Gesellschaft für Schmetterlingsschutz e.V, Halle
Leidner AK, Haddad NM (2010) Natural, not urban, barriers define population structure for a coastal endemic butterfly. Conserv Genet 11:2311–2320
Lund Municipality (2009) Program till detaljplan för Idalafältet (del av Veberöd 17:2 m.m) i Veberöd, Lunds kommun. (Program for detailed development plan for Idala field in Veberöd, Lund municipality). Stadsbygnadskontor
Maes D, Van Dyck H (2001) Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol Conserv 99:263–276
Marschalek DA, Deutschman DH (2008) Hermes copper (Lycaena [Hermelycaena] hermes: Lycaenidae): life history and population estimation of a rare butterfly. J Insect Conserv 12:97–105
Matteson KC, Langellotto G (2012) Evaluating community gardens as habitat for an urban butterfly. Cities Environ CATE 5(1):10. http://digitalcommons.lmu.edu/cate/vol5/iss1/10
Matteson KC, Taron DJ, Minor ES (2012) Assessing citizen contributions to butterfly monitoring in two large cities. Conserv Biol 26:557–564
Meehan TD, Glassberg J, Gratton C (2013) Butterfly community structure and landscape composition in agricultural landscapes of the central United States. J Insect Conserv 17:411–419
New TR, Sands DPA (2002) Conservation concerns for butterflies in urban areas of Australia. J Insect Conserv 6:207–215
Nilsson SG, Franzén M, Petterson LB (2013) Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in southern Sweden. Nat Conserv 6:31–48
Öckinger E, Hammarstedt O, Nilsson SG, Smith HG (2006) The relationship of local extinctions of grassland butterflies and soil nitrogen levels. Biol Conserv 128:564–573
Öckinger E, Dannestam Å, Smith GH (2009) The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landsc Urban Plan 93:31–37
Pleasants JM, Oberhauser KS (2013) Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv Divers 6(2):135–144
Polus E, Vandewoestijne S, Choutt J, Baguette M (2007) Tracking the effects of one century of habitat loss and fragmentation on calcareous grassland butterfly communities. Biodivers Conserv 16:3423–3436
Preston KL, Redak RA, Allen MF, Rotenberry JT (2012) Changing distribution patterns of an endangered butterfly: linking local extinction patterns and variable habitat relationships. Biol Conserv 152:280–290
Restrepo LR, Halffter G (2013) Butterfly diversity in regional urbanization mosaic in two Mexican cities. Landsc Urban Plan 115:39–48
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Schlumprecht H, Bräu M (2013) Dukatenfalter (Lycaena virgaureae). In: Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (eds) Tagfalter in Bayern. Verlag Eugen Ulmer, Stuttgart, pp 196–198
Schneider C, Dover J, Fry GLA (2003) Movement of two grassland butterflies in the same habitat network: the role of adult food resources and size of the study area. Ecol Entomol 28:219–227
Settele J, Feldmann R, Reinhardt R (eds) (2000) Die Tagfalter Deutschlands. Verlag Eugen Ulmer, Stuttgart
StatSoft (2013) Statistica Release 12. StatSoft Inc, Tulsa, US
Strausz M, Fiedler K, Franzén M, Wiemers M (2012) Habitat and host plant use of the Large Copper Butterfly Lycaena dispar in an urban environment. J Insect Conserv 16:709–721
Streitberger M, Hermann G, Kraus W, Fartmann T (2012) Modern forest management and the decline of the Woodland Brown (Lopinga achine) in Central Europe. For Ecol Manag 269:239–248
Swengel SR, Schlicht D, Olsen F, Swengel AB (2011) Declines of prairie butterflies in the midwestern USA. J Insect Conserv 15:327–339
Thomas CD, Abery JCG (1995) Estimating rates of butterfly decline from distribution maps: the effect of scale. Biol Conserv 73:59–65
Tolman T, Lewington H (1997) Field guide butterflies of Britain and Europe. Harper Collins, London
Van Dyck H, Van Strien AJ, Maes D, Van Swaay CAM (2009) Declines in common, widespread butterflies in a landscape under intense human use. Conserv Biol 23:957–965
Van Swaay C, Warren M, Lois G (2006) Biotope use and trends of European butterflies. J Insect Conserv 10:189–209
Wallisdevries MF, Van Swaay CAM (2006) Global warming and excess nitrogen may induce butterfly decline by microclimatic cooling. Glob Chang Biol 12:1620–1626
Wallisdevries MF, Van Swaay CAM, Plate CL (2012) Changes in nectar supply: a possible cause of widespread butterfly decline. Curr Zool 58:384–391
Wenzel M, Schmitt T, Weitzel M, Seitz A (2006) The severe decline of butterflies on western German calcareous grasslands during the last 30 years: a conservation problem. Biol Conserv 128:542–552
Wood BC, Pullin AS (2002) Persistence of species in a fragmented urban landscape: the importance of dispersal ability and habitat availability for grassland butterflies. Biodivers Conserv 11:1451–1468
Acknowledgments
This study was funded by the Swedish University of Agricultural Sciences, Department of Landscape Architecture, Planning and Management, Alnarp. I am thankful to Mary McAfee, who corrected the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haaland, C. Abundances and movement of the Scarce Copper butterfly (Lycaena virgaureae) on future building sites at a settlement fringe in southern Sweden. J Insect Conserv 19, 255–264 (2015). https://doi.org/10.1007/s10841-014-9708-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9708-7