Abstract
Background
First experiences using a 64-electrode mini-basket catheter (BC) paired with an automatic mapping system (Rhythmia™) for catheter ablation (CA) of ventricular ectopy (VE) and ventricular tachycardia (VT) have been reported.
Objectives
We aimed to evaluate (1) differences in ventricular access for the BC and (2) benefit of this technology in the setting of standard clinical practice.
Methods
Patients (pts) undergoing CA for VE or VT using the Intellamap Orion™ paired with the Rhythmia™ automated-mapping system were included in this study. For LV access, transseptal and retrograde access were compared.
Results
All 32 pts (29 men, age 63 ± 15 years) underwent CA for VE (17 pts) or VT (15 pts). For mapping of VE originating from the left ventricle (LV) in 10 out of 13 pts, a transaortic access was feasible. The predominant access for CA of VT was transaortic (5/7). Feasibility and safety seem to be equal. The total procedure time was 179.1 ± 21.2 min for VE ablation and 212.0 ± 71.7 min for VT ablation (p = 0.177). For VE, an acquisition of 1602 ± 1672 map points and annotation of 140 ± 98 automated mapping points sufficed to abolish VE in all pts. During a 6-month follow-up (FU) after CA for VE, a VE burden reduction from 18.5 ± 2.1% to 2.8 ± 2.2% (p = 0.019) was achieved. In VT pts, one patient showed recurrence of sustained VT episodes during FU.
Conclusion
Use of a high-resolution mapping system for VE/VT CA potentially facilitates revelation of VE origin and VT circuits in the setting of standard clinical practice. Feasibility and safety of a venous, transaortic, transseptal, or a combined approach seem to be equal.
Similar content being viewed by others
1 Introduction
Catheter ablation (CA) of ventricular tachycardia (VT) has advanced as a safe and well-established treatment option with a remarkable suppression of VT recurrence [1, 2]. Comparable findings have been reported for ventricular ectopy (VE) [3, 4]. The right and left ventricular outflow tracts (RVOT/LVOT) are the most common sites of idiopathic VE. Therefore, CA is recommended as first-line therapy in those patients (RVOT) [5]. Several studies have proven the superiority of CA over the escalation of antiarrhythmic drug therapy (AAD) [1, 2, 4]. Therefore, CA of VT is recommended for patients with internal cardioverter defibrillator (ICD) with recurrent and/or incessant VT or VT storm [4]. Early CA not only improves quality of life, it furthermore reduces mortality and decrease ICD interventions and heart failure hospitalization rates [3, 6]. However, success rates of CA in VT vary due to the high complexity of the VT substrate and comorbidities of patients with structural heart disease along with possible procedure-related complications [2, 7]. To achieve a favorable procedure outcome, it is desirable to have a fast and thorough understanding of the VE origin or VT circuits. Thus, high-resolution mapping is thought to have a substantial impact on CA success rates for VE and VT [8]. The benefit of using a high-resolution map to reveal VT circuits in a swine animal model was reported earlier [9]. First acute in man experiences using a 64-electrode mini-basket catheter (BC) paired with a novel automatic mapping system (Rhythmia™, Boston Scientific Corporation, Cambridge, MA) for CA of VE and VT have been reported recently [10, 11].
The high-resolution mapping potentially facilitates identification of VE origin and VT circuits in the setting of sparse occurrence of VE or if induced VT is non-sustained or not hemodynamically tolerated. Furthermore, this study assessed potential differences in safety and efficacy of retrograde and antegrade access to the left ventricle (LV) for the BC.
2 Methods
2.1 Inclusion and exclusion criteria
We analyzed 32 consecutive patients with symptomatic VE or VT undergoing CA between 2016 and 2018 at the University Heart Centre of Cologne, Department of Electrophysiology, Germany. In all patients, the INTELLAMAP ORION™ Mapping Catheter (Boston Scientific Corporation, Cambridge, MA) in combination with the Rhythmia™ mapping system (Boston Scientific Corporation, Cambridge, MA) was used. Written informed consent was obtained in all cases as part of clinical routine. The study was approved by the local ethics’ board (ethical review board number, 17–440) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
2.2 Intraprocedural management
All 32 procedures were performed under deep sedation using midazolam, fentanyl, and propofol. Ablation of VE was performed, when tolerated by the patient, under local anesthesia. In patients with atrial fibrillation and intended transseptal or transaortic access to the LV, a transesophageal echocardiography was performed prior to the procedure to exclude left atrial thrombi. To provoke VE or induce VT, a quadripolar diagnostic catheter (Inquiry™, 5 French, Fa. Abbott, USA) was positioned within the right ventricle (RV) at the beginning of the procedure and a programmed ventricular stimulation (PVS) with up to three extra stimuli was performed. In addition, a decapolar diagnostic catheter (Inquiry™, 6 French, Fa. Abbott, USA) was placed in the coronary sinus as an annotation and system reference.
Isoproterenol was administered if VE remained non-inducible. A transaortic access to the LV via right femoral artery or a single transseptal puncture was performed based on operator’s assessment and the assumed localization of arrhythmia. Transseptal puncture was accomplished under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5F transseptal sheath (SL1, Fa. Abbott, Inc., St. Paul, MN, USA,). After transseptal puncture, the sheath was changed to an 8.5F steerable sheath (Agilis NxT, St. Fa. Abbott, Inc., St. Paul, MN, USA). After transaortic access or transseptal puncture, heparin boluses were administered targeting an activated clotting time of 300–350 s. All procedures were performed under continuous hemodynamic monitoring. In case of a preexisting ICD or pacemaker, a device interrogation was conducted before and after the procedure.
2.3 Rhythmia mapping
All maps were exclusively acquired from the endocardial surface using the BC. In patients with VE, activation and pace maps were acquired using the systems automatic beat template matching for VE. The Rhythmia™ mapping system (Boston Scientific Corporation, Cambridge, MA) acquires automatically geometry and electrograms for voltage and timing annotation. In all patients undergoing RF CA for VE, the VE morphology was automatically identified using the defined beat acceptance criteria. The following criteria were used for beat acceptance: cycle length (for VT only), respiration gating, stable catheter position, aEGM stability (for VT only), QRS morphology, and tracking quality. Ablation for VE was performed at the site of the earliest activation as annotated by the automatic activation map using the aECG algorithm. If earliest activation was inconclusive, pace-mapping information was additionally used to determine the source of ectopic activation. Induction of VT was performed using PVS and if VT was stable and hemodynamically tolerated an automated activation and substrate mapping was obtained during VT. Noise level and complete electrical silence were considered as a voltage amplitude < 0.03 mV. In LV voltage maps, the standard voltage cutoff value of < 1.5 mV was applied. In 25 out of 32 (78%) patients, a Thermocool™ D or F-Curve (Biosense Webster Inc., CA, US; power 25–60 W, temperature limited to 45 °C), was used. In seven patients (22%), an INTELLANAV™ Ablation Catheter, (Boston Scientific Corporation, Cambridge, MA was used) was used. To identify potential VT isthmus and ablation site, stimulus to QRS onset, pace-mapping, and VT-entrainment [12, 13] were taken into account. To obtain a thorough ablation of the VT substrate, late abnormal ventricular activation was targeted and abolished. Ablation endpoint was non-inducibility of VT or complete abolishment of targeted VT. The endpoint for VE was reached when no VE occurred, even after a waiting time of 30 min and after provocation with isoproterenol. All interventions were performed by two experienced electrophysiologists who performed at least 800 CA per year.
2.4 Post-procedural care
Closure of the arterial puncture site was accomplished using the Angio-Seal®-system (Terumo interventional systems, Europe NV). ICDs were reprogrammed to the initial parameters at the end of the procedure. Continuous electrocardiographic telemetric monitoring was performed for at least 24 h after the ablation procedure to detected potential early recurrence of VE or VT. In patients undergoing CA for VE, AADs were discontinued. Beta blockers in VE were only continued if indicated for congestive heart failure (CHF) or arterial hypertension. Same applies for patients after CA for idiopathic VT. In case of CA for VT, patients continued AADs for 3 months for stabilization after ablation if tolerated. Patients were discharged 48 h after the procedure.
2.5 Clinical follow-up and study endpoints
During follow-up (FU), all outpatient clinic visits at 6 months including ECGs, 24 h-Holter electrograms and device interrogation if present were planned. In case of preexisting telemonitoring via implanted loop recorder, additional database interrogation was performed. Immediate outpatient clinic visits were initiated if patients reported symptoms suggestive of an arrhythmia relapse. The primary endpoint was defined as recurrence of any symptomatic or documented sustained VT or VE during FU. Secondary endpoints were acute procedural parameters (procedure duration, mapping points, duration of RF delivery, and delivered RF energy) and procedure-related complications such as cerebral embolism, pericardial effusion, or pericardial tamponade. The procedure time was defined from femoral puncture until removal of femoral sheaths. Furthermore, feasibility and complications of different left ventricular access routes were evaluated.
2.6 Statistical analysis
Differences of metric variables (baseline characteristic) between VE and VT and between different access routes (retrograde vs. transseptal) patients were analyzed with t test if the data were normally distributed, and with Wilcoxon-Mann–Whitney U test otherwise. Differences between categorical variables were evaluated using the chi-square test or the Fisher’s exact test in case of small expected cell frequencies. All p values are two-sided and a p value < 0.05 was considered significant.
3 Results
3.1 Study population
The baseline characteristics of the study population are presented in Table 1. A total of 32 consecutive patients (29 male, mean age 63 ± 15 years) underwent successful endocardial RF CA for VE/VT. All patients were hemodynamically stable before the procedure. Of these 32 patients, 17 patients (15 male, mean age 59.2 ± 13.4 years) underwent RF CA for VE and 15 for VT (14 male, mean age 66 ± 17 years). The origin of the VE/VT was located in the LV in 28/32 patients (87.5%).
3.2 VE patients: study population
The VE burden before CA was 18.5 ± 2.1%. Three VE patients had an ICD and one patient had an implanted event recorder. Relevant history of VE patients included ischemic cardiomyopathy in nine out of 17 patients (52.9%). Two patients (11.7%) presented a history of myocarditis with current normal cardiac function. Six patients (35.2%) showed no structural heart disease. The AAD regime consisted of amiodarone in one (5.9%) VE patient. Beta blockers were taken by 16 VE patients (94.1%). In only six out of these 16 patients (37.5%), beta blockers were used solely as an AAD.
3.3 VT patients: study population
All patients suffered from recurrent VTs and no patient met criteria of an electrical storm. Ten VT patients presented with an ICD, one patient with a pacemaker and one with an implanted event recorder. All VT patients without ICD showed a preserved LVEF. Relevant history included coronary artery disease in 13 out of 15 patients (86.7%). Ten out of 15 (66.7%) patients were on amiodarone. All patients with a reduced LVEF were on an optimized guideline-based heart failure medication composed of angiotensin-converting enzyme inhibitors (ACE-Inhibitor) or sacubitril/valsartan, beta blockers, diuretics, and mineralocorticoid receptor antagonists (Table 1).
3.4 Procedural characteristics
All 32 patients underwent successful endocardial RF CA of VT/VE. The procedure data are presented in Table 2.
3.5 Access to the left ventricle in VE patients
In four patients, the origin of the VE was located in the RV. For mapping of the LV in 10 out of 13 VE patients (76.9%), a transaortic access was chosen. For two patients, a transseptal approach was performed due to severe aortic kinking and elongation of latter. In one patient, a transseptal approach was performed after failed transaortic passage of the aortic valve. The procedure duration in VE patients did not differ significantly regarding the chosen access (procedure time for VE with a transseptal access 173.3 ± 63.5 min, procedure time for VE using transaortic access 185.6 ± 323.8 min, p = 0.8). During mapping via transseptal and transaortic access, the BC was able to reach all areas of the LV needed for CA. There were no complications related to the BC after the aortic valve passage. Furthermore, the BC did not perturb the mitral valve function during or after the procedure.
3.6 Access to the left ventricle in VT patients
The predominant access for CA of VT was transaortic in 11 out of 15 patients (73.4%). For four out of 15 VT patients (26.6%), a transseptal access was performed due to aortic valve replacement. No differences were seen regarding safety and efficacy of either approach (Table 2). The procedure duration did not show significant differences between a transseptal or a transaortic access (procedure time VT using a transseptal access 233.0 ± 45.1 min, procedure time VT using a transaortic access 229 ± 32.6 min, p = 0.89). Independent of the chosen access, no device dysfunction occurred in case of preexisting implanted devices (pacemaker/internal cardioverter defibrillator). During mapping via transseptal and transaortic access, the BC was able to reach all areas of the LV needed for CA. There were no complications related to the BC after the aortic valve passage. Furthermore, the BC did not perturb the mitral valve function during or after the procedure.
3.7 Treatment of ventricular ectopy
Occurrence of sparse spontaneous VE (< 1 VE/min) at the time of the procedure was seen in 10 out of 17 patients (58.8%) despite provocation with isoproterenol, hand-grip exercise, or PVS. During pace mapping, the template match was > 98% in all patients with VE. A total of 1.2 ± 0.4 maps/patient were obtained with the Rhythmia™ mapping system (Boston Scientific Corporation, Cambridge, MA). The total procedure time in all patients undergoing CA for VE was 179.1 ± 321.2 min. The procedure duration in VE patients including mapping and ablation in the RV only was 162.0 ± 35.0 min. For VE, an acquisition of 1602 ± 1672 map points and annotation of 140 ± 98 aECG points sufficed to abolish VE in all patients. The ablation endpoint of no inducibility or possible provocation of VE was reached in all patients. A mean of 12.0 ± 10.1 impulses of RF energy with a mean energy of 30,342.7 ± 21,877.9 J were delivered to reach the endpoint of VE abolishment (Fig. 1). A mean of 1.2 ± 0.4 VE morphologies were targeted. Distribution of VE origin included the right and left ventricular outflow tract and the LV (Table 2). One patient presented a post-procedural femoral hematoma after arterial puncture. The hematoma was treated conservatively. No blood transfusion or intervention was required or prolonged the hospital stay. No late complication of the hematoma was noted. The same applies to one venous hematoma after femoral puncture. Furthermore, no complications occurred in any patient undergoing CA for VE.
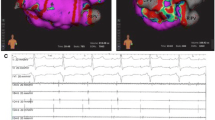
A map acquired during an ablation procedure of ventricular ectopy with sparse occurrence of ventricular ectopy from the left ventricle. The origin of the earliest activation is displayed (white arrow) with black arrows indicating the propagation wave front. The purple color indicates a voltage value of 1.5–20 mV. After ablation with two applications of radiofrequency energy, a complete suppression of the ventricular ectopy was achieved at this point
3.8 Treatment of ventricular tachycardia
In 10/15 patients (66.7%), induced VT was either non-sustained or hemodynamically not tolerated. The total procedure time for RF CA for VT was 212.0 ± 71.7 min. A high-resolution substrate map of 7980 ± 3132 points was achieved in all VT patients, despite analyzing only short VT runs. The ablation endpoint of non-inducibility of VT was reached in all patients. A mean of 33.9 ± 22.8 impulses of RF energy with a mean energy of 202,362 ± 345,390 J was delivered to reach the endpoint. A mean of 1.6 ± 0.9 VT morphologies was treated. The origin of VTs in all VT patients was located in the LV (Table 2). Figure 2 presents an example of a propagation map for a VT, which could be successfully ablated in the LV. Furthermore, the critical VT Isthmus was revealed using the mapping system and confirmed by long stimulus to QRS onset interval and successful abolishment of VT at the isthmus side. Ablation of critical isthmus sites was continued until non-excitability of targeted region. Two patients suffered from a femoral hematoma. These two patients had arterial femoral access. These hematomas were treated conservatively. No blood transfusion or intervention was required or prolonged the hospital stay. No late complication of the hematoma was noted. Furthermore, no complications occurred in any patients undergoing CA for VT.
a–f A propagation map of a ventricular tachycardia located in the left ventricle in a patient with impaired hemodynamics during VT, therefore only short mapping episodes during VT were possible. However, the series of images reveal the VTs’ critical isthmus followed by the propagating VT wave front activating the LV. The region of the earliest activation is red and the region of the latest activation is colored blue. The gray dots marks the VT isthmus. This region was subsequently ablated until non-excitability the endpoint of no inducibility of the ventricular tachycardia was reached after ablation. g A voltage map of the same LV with corresponding voltage information to the detected critical isthmus in the propagation map. The critical isthmus was confirmed by long stimulus to QRS onset interval. The purple color shows a voltage of 1.0–20 mV. The red color presents scar with a voltage < 0.09 mV
3.9 VE patients: follow-up and success rate
During a 6-month FU after CA for VE, a significant VE burden reduction from 18.5 ± 2.1 to 2.8 ± 2.2% (p = 0.019) was achieved. Post-procedural amiodarone medication was already suspended in all patients for more than 6 weeks (Table 3). Up to this time, no re-procedure in the VE group was necessary. One patient of the VE group died after 6 months due to a fulminant non-procedure-related cerebral stroke along with terminal heart failure.
3.10 VT patients: follow-up and success rate
During FU of 6 months, one VT patient showed occurrence of sustained VT episodes. In two patients, non-sustained, asymptomatic VTs occurred during Holter-ECG and device interrogation. No further complications occurred during the 6-month FU. In patients treated for VT, the AAD consisted of amiodarone in three patients (20.0%) at 6 months FU (Table 3). The amiodarone medication in VT patients with sustained or non-sustained VT was continued. In patients with no occurrence of VT, amiodarone was interrupted in case of a preexisting amiodarone medication.
4 Discussion
The use of a 64-pole BC paired with an automatic mapping system (Rhythmia™) for CA of VE and VT has been implemented into clinical routine. In this study, we sought to evaluate possible advantages and risks of different ventricular accesses. Moreover, we evaluated the systems potential additional benefit to VE and VT ablation especially in the setting of challenging conditions. The main findings of our study are:
-
1)
No differences were seen regarding safety, efficacy, or feasibility between a transseptal or transaortic LV access. The BC was able to reach all areas of the LV and no complications occurred which were related to the BC.
-
2)
The use of a high-resolution mapping system for VE/VT ablation facilitates revelation of VE origin and VT circuits resulting in satisfactory acute and long-term success rates.
4.1 Access to the left ventricle
In previous studies, no detailed information was provided regarding the left ventricular access. However, it is known that both approaches, transseptal and transaortic, were used to reach the LV using the BC [7]. Whether one of the two accesses was more efficient or safe is not fully clarified yet. Regarding efficacy or safety of either access, no clear benefit was observed, however in three cases, retrograde access via the aortic valve was not possible. For two patients, a transseptal approach was performed due to severe aortic kinking and elongation. Furthermore, in one patient, a switch to transseptal access was necessary after a failed transaortic attempt. Although providing a bidirectional curve of 180° and a deployment range of 3–22 mm, no transaortic access to the LV was feasible in these three cases. This shows that in some cases the BC catheter positioning can be difficult during a standard transaortic access to the LV, most likely because of its rigidity and size. Therefore, early transseptal access may facilitate the procedure and lower complication risks. Pluta et al. reported in 2010 on similar success rates in transseptal versus retrograde groups in patients undergoing VT CA. But the procedure time was longer in patients with transaortic access to the LV (112 min for transseptal vs. 145 min for retrograde approach) [14]. Tilz et al. reported 2014 on similar mapping times for both approaches (24 ± 9.2 min for transseptal vs. 30 ± 91 min for retrograde approach, p = 0.16) [15]. We were able to show that a transseptal access is an accurate, safe, feasible, and effective method for BC mapping and RF CA in patients with left ventricular arrhythmias. Despite this fact, our predominant access was transaortic. Interestingly, the procedure duration did not differ significantly between the transseptal and transaortic access in the VE and VT patients in our study population. Both approaches seem to be equal regarding feasibility and safety. However, our findings have to be confirmed in larger randomized studies.
4.2 Catheter ablation for VE and VT
Sparse VE occurrence potentially leads to impaired abolishment of latter. Also, hemodynamic instability and lack of inducibility may hinder successful CA of VT. These limitations are seen in a relevant number of patients in daily routine [6]. Several algorithms and strategies to improve CA results have been developed and evaluated [16]. Nevertheless, it is desirable to have a safe, fast, and efficient mapping and ablation strategy for those patients. Novel technologies like the high-resolution mapping systems may offer the opportunity to rapidly reconstruct a ventricular electroanatomic map in detail and identify VE origin and VT circuits and therefore may shorten the procedure time along with increased success rates. First, small studies and [10, 11] few case reports [17, 18] revealed promising results on safety and feasibility using this novel technology for the treatment of patients with unstable VT.
Takigawa et al. reported on a case of an unstable hemodynamic VT in which the circuit was found in 35 s using the Rhythmia™ mapping system. These findings correspond with our experience that the mapping time can be shortened dramatically using the Rhythmia™ mapping system as compared to the experience with conventional mapping systems [18]. Despite this short mapping time, the maps are of high-resolution and reveal novel insights to VT circuits (Fig. 1). Due to the BC with 64 electrodes, a large number of mapping points can be taken within a short time period.
4.3 Comparison to other 3D mapping systems
An interesting question is the comparison of the Rhythmia™ mapping system with other 3D mapping systems in terms of mapping time. Maagh et al. reported on a single-center VT study from 2012 to 2014 using a multi-electrode mapping catheter in combination with a 3D mapping system. The average mapping time was 55.6 ± 34.4 min [19]. It appears that the mapping time is comparable with the Rhythmia™ mapping system in comparison to other 3D mapping systems.
Wolf et al. reported on outcomes of substrate elimination targeting local abnormal ventricular activities (LAVA) for post-myocardial infarction VT [20]. Three-dimensional electroanatomical mapping systems, multi-electrode mapping catheters, and real-time image integration were used. Mapping was either performed with a 3.5-mm open-irrigated ablation catheter (Biosense Webster, Diamond Bar, CA) or preferably with a multipolar high-density mapping catheter (Pentaray 2-6-2-mm spacing, Biosense Webster, or Intellamap Orion, Boston Scientific). The total procedure duration was 247.9 ± 78.2 min. Similar procedure times (229 ± 66 min) using a 3D mapping system have been reported by Andreu et al. [21]. The findings of Wolf and Andreu are comparable with our data (procedure duration VT: 212.0 ± 71.7 min).
4.4 Safety
Our data showed a satisfactory outcome in terms of safety. It is noteworthy that no patient from our study population required pharmacological and mechanical hemodynamic support during or after the procedure. Although VT patients are a high-risk group, due to heart failure, possible rapid cardiac impairment during or after the procedure, no transfer to intensive care was necessary for any patient. However, it should be mentioned that our study population consists of more patients with VE with a rather preserved LVEF than VT patients with severely impaired LVEF. Nevertheless, our procedure data are well in line with previous publications regarding safety aspects using a high-resolution mapping system [10, 11].
4.5 Limitations
First, this is a non-randomized study with a small sample size. However, we report 32 consecutive patients describing the usage of this new technology in a setting of standard clinical practice. Nevertheless, our findings have to be confirmed in a larger study population. Second, there were no pre-specified protocols for VT/VE-mapping. However, standard mapping and ablation protocols comparable to the usage of other mapping systems in our daily routine were followed.
5 Conclusion
The use of a high-resolution mapping system for VE/VT ablation possibly facilitates revelation of VE origin or VT circuits resulting in satisfactory acute and long-term success rates. Both, a transaortic and a transseptal approach are secure for BC introduction and mapping. Our data have to be confirmed in larger prospective studies.
Abbreviations
- CA:
-
Catheter ablation
- VT:
-
Ventricular tachycardia
- VE:
-
Ventricular ectopy
- BC:
-
Mini-basket catheter
- LVEF:
-
Left ventricular ejection fraction
- ICD:
-
Internal cardioverter defibrillator
- LV:
-
Left ventricle
- RV:
-
Right ventricle
- RF:
-
Radiofrequency
- PVS:
-
Programmed ventricular stimulation
- FU:
-
Follow up
- AECG:
-
Automated electrograms
- AAD:
-
Antiarrhythmic drug therapy
- CHF:
-
Congestive heart failure
References
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for practice guidelines. J Am Coll Cardiol. 2006;48:247–346.
Sacher F, Tedrow UB, Field ME, Raymond JM, Koplan BA, Epstein LM, et al. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008;1:153–61.
Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the prospective Heart Centre of Leipzig VT (HELP-VT) study. Circulation. 2014;129:728–36.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm Society. J Am Coll Cardiol. 2018;72:e91–220.
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867.
Santangeli P, Frankel DS, Marchlinski FE. End points for ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7:949–60.
Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65.
Tung R, Mathuria NS, Nagel R, Mandapati R, Buch EF, Bradfield JS, et al. Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol. 2013;6:1131–8.
Tschabrunn CM, Roujol S, Dorman NC, Nezafat R, Josephson ME, Anter E. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 2016;9:10.
Viswanathan K, Mantziari L, Butcher C, Hodkinson E, Lim E, Khan H, et al. Evaluation of a novel high-resolution mapping-system for catheter ablation of ventricular arrhythmias. Heart Rhythm. 2017;14:176–83.
Mantziari L, Butcher C, Kontogeorgis A, Panikker S, Roy K, Markides V, et al. Utility of a novel rapid high-resolution mapping-system in the catheter ablation of arrhythmias. JACC: Clini Electrophysiol. 2015;1:411–20.
Fernández-Armenta J, Penela D, Acosta J, Andreu D, Evertz R, Cabrera M, et al. Substrate modification or ventricular tachycardia induction, mapping, and ablation as the first step? A randomized study. Heart Rhythm. 2016;13:1589–95.
Martínez-Rubio A, Kuschyk J, Sierra G, Breithardt G, Borggrefe M. Programmed ventricular stimulation: influence of early versus late introduction of a third extrastimulus, a randomized, prospective study. Europace. 2002;4:77–85.
Pluta S, Lenarczyk R, Pruszkowska-Skrzep P, Kowalski O, Sokal A, Sredniawa B, et al. Transseptal versus transaortic approach for radiofrequency ablation in patients with cardioverter-defibrillator and electrical storm. J Interv Card Electrophysiol. 2010;28:45–50.
Tilz RR, Makimoto H, Lin T, Rillig A, Metzner A, Mathew S, et al. In vivo left-ventricular contact force analysis: comparison of antegrade transseptal with retrograde transaortic mapping strategies and correlation of impedance and electrical amplitude with contact force. Europace. 2014;16:1387–95.
Steven D, van den Bruck JH, Lüker J, Plenge T, Sultan A. 3-D mapping of ventricular tachycardia in patients with dilative cardiomyopathy. Herzschrittmacherther Elektrophysiol. 2017;28:206–11.
Kaiser L, Jularic M, Akbulak RÖ, Nührich J, Willems S, Meyer C. Catheter ablation of hemodynamically unstable ventricular tachycardia in ischemic cardiomyopathy using high-resolution mapping. Clin Case Rep. 2017;5:389–93.
Takigawa M, Frontera A, Thompson N, Capellino S, Jais P, Sacher F. The electrical circuit of a hemodynamically unstable and recurrent ventricular tachycardia diagnosed in 35 s with the Rhythmia mapping-system. J Arrhythm. 2017;33:505–7.
Maagh P, Christoph A, Müller MS, Dopp H, Plehn G, Meissner A. Point-by-point versus multisite electrode mapping in VT ablation: does freedom from VT recurrences depend on mapping catheter? An observational study. J Interv Card Electrophysiol. 2018;51:169–81. https://doi.org/10.1007/s10840.
Wolf M, Sacher F, Cochet H, Kitamura T, Takigawa M, Yamashita S, et al. Long-term outcome of substrate modification in ablation of post-myocardial infarction ventricular tachycardia. Circ Arrhythm Electrophysiol. 2018;11:e005635. https://doi.org/10.1161/CIRCEP.117.005635.
Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias D, et al. Cardiac magnetic resonance-aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm. 2017;14:1121–8.
Author information
Authors and Affiliations
Contributions
Arian Sultan, MD: concept/design, data analysis/interpretation, drafting article, statistics, data collection.
Barbara Bellmann, MD: concept/design, data analysis/interpretation, drafting article, statistics, data collection.
Jakob Lüker, MD: data analysis/interpretation, critical revision of article, data collection.
Tobias Plenge, MD: critical revision of article, data collection.
Jan-Hendrik van den Bruck: critical revision of article, data collection.
Karlo Filipovic, MD: critical revision of article, data collection.
Susanne Erlhöfer, MD: critical revision of article, data collection.
Liz Kuffer, MD: critical revision of article, data collection.
Zeynep Arica: critical revision of article, data collection.
Daniel Steven, MD: concept/design, data analysis/interpretation, data collection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- No differences were seen regarding safety, efficacy, or feasibility between a transseptal or transaortic LV access. The BC was able to reach all areas of the LV needed for CA and no complications occurred which were related to the BC.
- The use of a high-resolution mapping system for VT/VE ablation facilitates revelation of VT circuits or VE origin in the setting of challenging mapping conditions resulting in favorable acute and long-term success rates.
Rights and permissions
About this article
Cite this article
Sultan, A., Bellmann, B., Lüker, J. et al. The use of a high-resolution mapping system may facilitate standard clinical practice in VE and VT ablation. J Interv Card Electrophysiol 55, 287–295 (2019). https://doi.org/10.1007/s10840-019-00530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-019-00530-1