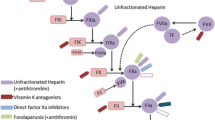
Abstract
The focus of this review is the evolving field of antithrombotic drug therapy for stroke prevention in patients with atrial fibrillation (AF). The current standard of therapy includes warfarin, acenocoumarol and phenprocoumon which have proven efficacy by reducing stroke by 68% against placebo. However, a narrow therapeutic index, wide variation in metabolism, and numerous food and drug interactions have limited their clinical application to only 50% of the indicated population. Newer agents such as direct thrombin inhibitors, factor Xa inhibitors, factor IX inhibitors, tissue factor inhibitors and a novel vitamin K antagonist are being developed to overcome the limitations of current agents. The direct thrombin inhibitor dabigatran is farthest along in development. Further clinical trial testing, and eventual incorporation into clinical practice will depend on safety, efficacy and cost. Development of a novel vitamin K antagonist with better INR control will challenge the newer mechanistic agents in their quest to replace the existing vitamin K antagonists. Till then, the large unfilled gap to replace conventional agents remains open. This review will assess all these agents, and compare their mechanism of action, stage of development and pharmacologic profile.



Similar content being viewed by others
References
Mueller, R. L., & Scheidt, S. (1994). History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation, 89, 432–449.
Shapiro, S. S. (2003). Treating thrombosis in the 21st century. New England Journal of Medicine, 349, 1762–1764.
Fasco, M. J., & Principe, L. M. (1982). R- and S- Warfarin inhibition of vitamin K and vitamin K 2,3-epoxide reductase activities in the rat. Journal of Biological Chemistry, 257, 4894–4901.
Hirsh, J., Dalen, J. E., Anderson, D. R., Poller, L., Bussey, H., Ansell, J., et al. (2001). Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest, 119(1 Suppl), 8S–21S (Jan).
Ansell, J., Hirsh, J., Poller, L., Bussey, H., Jacobson, A., & Hylek, E. (2004). The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest, 126(Suppl), 204S–233S.
Ufer, M. (2005). Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clinical Pharmacokinetics, 44, 1227–1246 Review.
Petersen, P., Boysen, G., Godtfredsen, J., Andersen, E. D., & Andersen, B. (1989). Placebo controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet, 1, 175–179.
Petersen, P., & Boysen, G. (1990). Stroke prevention in atrial fibrillation study. New England Journal of Medicine, 323, 482.
The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators (1990). The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation.. New England Journal of Medicine, 323, 1505–1511.
Stroke Prevention in Atrial Fibrillation Study Group (1991). Final results. Circulation, 84, 527–539.
Connolly, S. J., Laupacis, A., Gent, M., Roberts, R. S., Cairns, J. A., & Joyner, C. (1991). Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. Journal of the American College of Cardiology, 18, 349–355.
Ezekowitz, M. D., Bridgers, S. L., James, K. E., et al. (1992). Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. New England Journal of Medicine, 327, 1406–1412.
EAFT (European Atrial Fibrillation Trial) Study Group (1993). Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke.. Lancet, 342, 1255–1262.
Stroke Prevention in Atrial Fibrillation II Study Group (1994). Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet, 343, 687–691.
Morocutti, C., Amabile, G., Fattapposta, F., et al. (1997). Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke, 28, 1015–1021.
Gulløv, A. L., Koefoed, B. G., Petersen, P., et al. (1998). Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Archives of Internal Medicine, 158, 1513–1521.
Hellemons, B. S., Langenberg, M., Lodder, J., et al. (1999). Primary prevention of arterial thromboembolism in non-rheumatic atrial fibrillation in primary care: randomised controlled trial comparing two intensities of coumarin with aspirin. British Medical Journal, 319, 958–964.
Pérez-Gómez, F, Alegría, E, Berjón, J., Iriarte, J. A., Zumalde, J., Salvador, A, Mataix, L., & NASPEAF Investigators (2004). Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and nonvalvular atrial fibrillation: a randomized multicenter study. Journal of the American College of Cardiology, 44, 1557–1566.
Writing ACTIVE Group (2006). Group on behalf of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet, 367, 1903–1912.
Antithrombotic Therapy in Atrial Fibrillation Study Group (2006). [The randomized study of efficiency and safety of antithrombotic therapy in nonvalvular atrial fibrillation: warfarin compared with aspirin]. Zhonghua Xin Xue Guan Bing Za Zhi, 34, 295–298.
Mant, J., Hobbs, F. D., Fletcher, K., et al. (2007). BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet, 370, 493–503.
Hart, R. G., Pearce, L. A., & Aguilar, M. I. (2007). Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine, 146, 857–867.
Bungard, T. J., Ghali, W. A., Teo, K. K., McAlister, F. A., & Tsuyuki, R. T. (2000). Why do patients with atrial fibrillation not receive warfarin? Archives of Internal Medicine, 160, 41–46.
Bradley, B. C., Perdue, K. S., Tisdel, K. A., & Gilligan, D. M. (2000). Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. American Journal of Cardiology, 85, 568–572.
Siguret, V. (2007 ). Impact of pharmacogenetics on interindividual variability in the response to vitamin K antagonist therapy. Pathologie et Biologie (Paris), 55, 295–298 Article in French.
Ansell, J., Hirsh, J., Poller, L., Bussey, H., Jacobson, A., & Hylek, E. (2004). The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest, 126(Suppl), 204S–233S.
Carlquist, J. F., Horne, B. D., Muhlestein, J. B., et al. (2006). Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. Journal of Thrombosis and Thrombolysis, 22, 191–197.
Bauer, K. A. (2006). New anticoagulants. Hematology (Am Soc Hematol Educ Program), 450–456, DOI 10.1182/asheducation-2006.1.450.
Gage, B. F., Eby, C., Milligan, P. E., Banet, G. A., Duncan, J. R., & McLeod, H. L. (2004). Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thrombosis and Haemostasis, 91, 87–94.
Bates, S. M., & Weitz, J. I. (2000). The mechanism of action of thrombin inhibitors. Journal of Invasive Cardiology, 12(Suppl F), 27F–32F.
Weitz, J. I., Leslie, B., & Hudoba, M. (1998). Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation, 97, 544–552.
Olsson, S. B. (2003). Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet, 362, 1691–1698.
Albers, G. W., Diener, H. C., Frison, L., et al. (2005). SPORTIF Executive Steering Committee for the SPORTIF V Investigators. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA, 293, 690–698.
Akins, P. T., Feldman, H. A., Zoble, R. G., et al. (2007). Secondary stroke prevention with ximelagatran versus warfarin in patients with atrial fibrillation: pooled analysis of SPORTIF III and V clinical trials. Stroke, 38, 874–880.
Kaul, S., Diamond, G. A., & Weintraub, W. S. (2005). Trials and tribulations of non-inferiority: the ximelagatran experience. Journal of the American College of Cardiology, 46, 1986–1995.
Wallentin, L., Ezekowitz, M., Simmers, T. A., et al. (2005). PETRO-investigators. Safety and efficacy of a new oral direct thrombin inhibitor dabigatran in atrial fibrillation: a dose finding trial with comparison to warfarin. European Heart Journal, 26(suppl), 482 Abstract.
Gustafsson, D. (2003). Oral direct thrombin inhibitors in clinical development. Journal of Internal Medicine, 254, 322–334.
Mungall, D. (2002). BIBR-1048 Boehringer Ingelheim. Current Opinion in Investigational Drugs, 3, 905–907.
Ezekowitz, M. D., Reilly, P. A., Nehmiz, G., Simmers, T. A., Nagarakanti, R., Parcham-Azad, K., et al. (2007). Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). American Journal of Cardiology, 100(9), 1419–1426 (Nov 1).
The Petro-ex Investigators (2006). Safety and efficacy of extended exposure to several doses of a new oral direct thrombin inhibitor dabigatran etexilate in atrial fibrillation. Cerebrovascular Diseases, 21(suppl 4), 2 Abstract.
Bauer, K. A. (2006). New anticoagulants: anti IIa vs anti Xa—is one better? Journal of Thrombosis and Thrombolysis, 21, 67–72.
Perzborn, E., Strassburger, J., Wilmen, A., et al. (2005). In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor. Thrombosis and Haemostasis, 3, 514–521.
Mueck, W., Becka, M., Kubitza, D., Voith, B., & Zuehlsdorf, M. (2007). Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor xa inhibitor—in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics, 45, 335–344.
Kubitza, D., Becka, M., Wensing, G., Voith, B., & Zuehlsdorf, M. (2005). Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. European Journal of Clinical Pharmacology, 61, 873–880.
Kubitza, D., Becka, M., Zuehlsdorf, M., & Mueck, W. (2007). Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. Journal of Clinical Pharmacology, 47, 218–226.
Kubitza, D., Becka, M., Zuehlsdorf, M., & Mueck, W. (2006). Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct Factor Xa inhibitor, in healthy subjects. Journal of Clinical Pharmacology, 46, 549–558.
Pinto, D. J., Orwat, M. J., Koch, S., et al. (2007). Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. Journal of Medicinal Chemistry, 50, 5339–5356.
Lassen, M. R., Davidson, B. L., Gallus, A., Pineo, G., Ansell, J., & Deitchman, D. (2007). The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. Thrombosis and Haemostasis, 5, 2368–2375.
Koopman, M. M. W., & Buller, H. R. (2003). Short- and long-acting synthetic pentasaccharides (minisymposium). Journal of Internal Medicine, 254, 335–342.
Sanofi-aventis Press Release (2007). en.sanofi-aventis.com/Images/070711_idraparinux_en_tcm24-18621.pdf.
Agnelli, G., Haas, S., Ginsberg, J. S., Krueger, K. A., Dmitrienko, A., & Brandt, J. T. (2007). A phase II study of the oral factor Xa inhibitor LY517717 for the prevention of venous thromboembolism after hip or knee replacement. Thrombosis and Haemostasis, 5, 746–753.
Iwatsuki, Y., Shigenaga, T., Moritani, Y., et al. (2006) Biochemical and pharmacological profiles of YM150, an oral direct Factor Xa inhibitor. Blood, 108, Abstract 911.
Overvie R&D. (2003). Yamanouchi Pharmaceutical Co Ltd. Company Presentation.
Eriksson, B. I., Turpie, A. G., Lassen, M. R., et al. (2007). ONYX study group.A dose escalation study of YM150, an oral direct factor Xa inhibitor, in the prevention of venous thromboembolism in elective primary hip replacement surgery. Thrombosis and Haemostasis, 5, 1660–1665.
Zafar, M. U., Vorchheimer, D. A., Gaztanaga, J., et al. (2007). Antithrombotic effects of factor Xa inhibition with DU-176b: Phase-I study of an oral, direct factor Xa inhibitor using an ex-vivo flow chamber. Thrombosis and Haemostasis, 98, 883–888.
Furugohri, T., Honda, Y., Matsumoto, C., et al. (2004). Antithrombotic and hemorrhagic effects of DU-176b, a novel, potent and orally active direct Factor Xa inhibitor: a wider safety margin compared to heparins and warfarin. American Society of Hematology Annual Meeting Abstract, 104, 1851.
Fukuda, T., Matsumoto, C., Honda, Y., Sugiyama, N., Morishima, Y., & Shibano, T. (2004). Antithrombotic properties of DU-176b, a novel, potent and orally active direct Factor Xa inhibitor in rat models of arterial and venous thrombosis: comparison with fondaparinux, an antithrombin dependent Factor Xa inhibitor. American Society of Hematology Annual Meeting Abstract, 104, 1852.
Neels, J. G., van Den Berg, B. M., Mertens, K., et al. (2000). Activation of factor IX zymogen results in exposure of a binding site for low-density lipoprotein receptor-related protein. Blood, 96, 3459–3465.
Lawson, J. H., & Mann, K. G. (1991). Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. Journal of Biological Chemistry, 266, 11317–11327.
Ahmad, S. S., Rawala-Sheikh, R., & Walsh, P. N. (1989). Platelet receptor occupancy with factor IXa promotes factor X activation. Journal of Biological Chemistry, 264, 20012–20016.
Chow, F. S., Benincosa, L. J., Sheth, S. B., et al. (2002). Pharmacokinetic and pharmacodynamic modeling of humanized anti-factor IX antibody (SB 249417) in humans. Clinical Pharmacology and Therapeutics, 71, 235–245.
Dyke, C. K., Steinhubl, S. R., Kleiman, N. S., et al. (2006). First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation, 114, 2490–2497.
Rothlein, R., Shen, J. M., Naser, N., et al. (2005). TTP889, a Novel Orally Active Partial Inhibitor of FIXa Inhibits Clotting in Two A/V Shunt Models without Prolonging Bleeding Times. Blood, 106, Abstract 1886.
Steffel, J., Lüscher, T. F., & Tanner, F. C. (2006). Tissue factor in cardiovascular disease. Molecular mechanisms and clinical implications. Circulation, 113, 722–731.
Ragni, M., Cirillo, P., Pascucci, I., et al. (1996). Monoclonal antibody against tissue factor shortens tissue plasminogen activator lysis time and prevents reocclusion in a rabbit model of carotid artery thrombosis. Circulation, 93, 1913–1918.
Harker, L. A., Hanson, S. R., Wilcox, J. N., & Kelly, A. B. (1996). Antithrombotic and antilesion benefits without hemorrhagic risks by inhibiting tissue factor pathway. Haemostasis, 26(Suppl 1), 76–82 Review.
Himber, J., Kirchhofer, D., Riederer, M., Tschopp, T. B., Steiner, B., & Roux, S. P. (1997). Dissociation of antithrombotic effect and bleeding time prolongation in rabbits by inhibiting tissue factor function. Thrombosis and Haemostasis, 78, 1142–1149.
Pawashe, A., Golino, P., Ambrosio, G., et al. (1994). A monoclonal antibody against rabbit tissue factor inhibits thrombus formation in stenotic injured rabbit carotid arteries. Circulation Research, 74, 56–63.
Golino, P., Ragni, M., Cirillo, P., et al. (1998). Antithrombotic effects of recombinant human, active site-blocked factor VIIa in a rabbit model of recurrent arterial thrombosis. Circulation Research, 82, 39–46.
Riggs, J. R., Kolesnikov, A., Hendrix, J., et al. (2006). FVIIa inhibitors: A prodrug strategy to improve oral bioavailability. Bioorganic & Medicinal Chemistry Letters, 16, 2224–2228.
Zbinden, K. G., Obst-Sander, U., Hilpert, K., et al. (2005). Selective and orally bioavailable phenylglycine tissue factor/factor VIIa inhibitors. Bioorganic & Medicinal Chemistry Letters, 15, 5344–5352.
Himber, J., Refino, C. J., Burcklen, L., Roux, S., & Kirchhofer, D. (2001). Inhibition of arterial thrombosis by a soluble tissue factor mutant and active site-blocked factors IXa and Xa in the guinea pig. Thrombosis and Haemostasis, 85, 475–481.
Fingerle, J., Himber, J., Tschopp, T., Bunting, S., Steiner, B., & Riederer, M. A. (2001). Minimal bleeding risk after chronic application of factor VIIa inhibitor in a guinea pig model. Thrombosis and Haemostasis, Supplement:abstract #OC1766.
Szalony, J. A., Suleymanov, O. D., Salyers, A. K., et al. (2003). Administration of a small molecule tissue factor/factor VIIa inhibitor in a non-human primate thrombosis model of venous thrombosis: effects on thrombus formation and bleeding time. Thrombosis Research, 112, 167–174.
Lee, A., Agnelli, G., Buller, H., et al. (2001). Dose-response study of recombinant factor VIIa/tissue factor inhibitor recombinant nematode anticoagulant protein c2 in prevention of postoperative venous thromboembolism in patients undergoing total knee replacement. Circulation, 104, 74–78.
Giugliano, R. P., Wiviott, S. D., Stone, P. H., et al. (2007). ANTHEM-TIMI-32 Investigators. Recombinant nematode anticoagulant protein c2 in patients with non-ST-segment elevation acute coronary syndrome: the ANTHEM-TIMI-32 trial. Journal of the American College of Cardiology, 49, 2398–2407.
Shirk, R. A., & Vlasuk, G. P. (2007). Inhibitors of Factor VIIa/tissue factor. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1895–1900.
Shirk, R. A., & Vlasuk, G. P. (2007). Inhibitors of Factor VIIa/tissue factor. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1895–1900.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umer Usman, M.H., Raza, S., Raza, S. et al. Advancement in antithrombotics for stroke prevention in atrial fibrillation. J Interv Card Electrophysiol 22, 129–137 (2008). https://doi.org/10.1007/s10840-008-9210-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-008-9210-9