Abstract
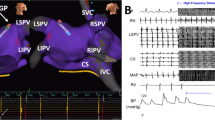
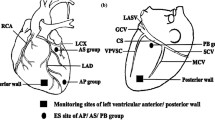
Experimental Studies: Anesthetized dogs were subjected to a right then left thoracotomy. Two modes of electrical stimulation were used to activate ganglionated plexi (GP) on the epicardium of the atria: (1) Near the base of each pulmonary vein (PV), trains of high frequency stimuli (HFS) were coupled to each atrial paced beat so as to fall within the refractory period to achieve nerve stimulation without atrial excitation; and (2) Continuous HFS was applied via plaque electrodes sutured to epicardial fat pads (containing a GP) near the right superior (RS) and left superior (LS) PVs. The chest was then closed. An ablation catheter, inserted percutaneously, was positioned fluoroscopically in the right atrium across from the epicardial plaque electrode near the RSPV. Transeptal puncture was used to place an ablation catheter at the LSPV-left atrial junction. HFS applied to each of the epicardial fat pads induced atrial fibrillation (AF) and also caused high grade AV block due to a strong parasympathetic effect on the AV node. Radiofrequency ablation from the right and left atrial endocardium abolished the vagal response to HFS delivered to the plaque electrodes on the fat pads close to the RSPV and LSPV, respectively.
Clinical Studies: Sixty (60) patients with paroxysmal or persistent AF underwent PV antrum isolation (27 patients) or PV antrum isolation plus left atrial GP ablation (33 patients). Endocardial HFS at the border of the PV antra near the 4 GPs produced AF and high grade AV block (vagal response) during AF. RFA at these sites abolished the vagal response. Testing in a small number of patients with very short follow-up suggests that adding GP ablation to PV antrum isolation may increase ablation success (absence of AF recurrence) from 70% to 91%.
Conclusions: These basic and clinical studies suggest that localized cardiac autonomic ganglia (GPs) may play a critical role in the initiation and maintenance of AF.
Similar content being viewed by others
References
Lewis T, Drury AN, Bulger HA. Observations upon atrial flutter and fibrillation. VI. Refractory period and rate of propagation in the auricle: Their relation to block in the auricular walls and to flutter etc. Heart 1921;8:84–134.
Hoff HE, Geddes LA. Cholinergic factor in atrial fibrillation. J Appl Physiol 1955;8:177–192.
Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol 1972;223:1068–1074.
Coumel P. Paroxysmal atrial fibrillation: A disorder of autonomic tone. Eur Heart J 1994;15:(Suppl A) 9–16.
Coumel P. Autonomic influence in atrial tachyarrhythmias. J Cardiovasc Electrophysiol 1996;7:999–1007.
Leenhardt A, Extramiana F, Cauchemez B, Musley SK, Foley L, Corrigan S. Role of anti-arrhythmics in the treatment of atrial fibrillation. Arch Mal Cocur Vaiss 2002;95:7–13.
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New Engl J Med 1998;339:659–666.
Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, Lazzara R, Jackman WM. Focal atrial fibrillation: Experimental evidence for a pathophysiological role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592–599.
Lazzara R, Scherlag BJ, Robinson MJ, Samet P. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ Res 1973;32:393–401.
Yuan B-X, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac neurons. Anat Rec 1994;239:75–87.
Armour JA, Murphy DA, Yuan B-X, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289–298.
Nakagawa H, Scherlag BJ, Aoyama H, et al. Catheter ablation of cardiac autonomic nerves for prevention of atrial fibrillation in a canine model (abstract). Heart Rhythm 2004;1:S10.
Randall WC, Ardell JL, Wurster RD, Milosavljevic M. Vagal postganglionic innervation of the canine sinoatrial node. J Auton Nerv System 1987;20:13–23.
Furukawa Y, Wallick DW, Carlson MD, Martin P. Prolongation of cardiac cycle length attenuates negative dromotropic response to selective vagal stimuli. J Auton Nerv Syst 1989;28:43–50.
Scherlag BJ, Yamanashi WS, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol 2005;45:1878–1886.
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J am Coll Cardiol 2004;43:2044–2053.
Platt M, Mandapati R, Scherlag BJ, et al. Limiting the number and extent of radiofrequency applications to terminate atrial fibrillation and subsequently prevent its inducibility (abstract). Heart Rhythm 2004;1:S11.
Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327–334.
Sharifov OF, Fedorov VV, Beloshajeko GG, Iushmanova AV, Rozenshtraukh LV. Isoproterenol potentiates atrial fibrillation induced by acetylcholine. Ross Fiziol Su Im IM Sechonova 2001;87:1296–1308.
Butler CK, Smith FM, Cardinal R, Murphy DA, Hopkins DA, Armour JA. Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. Am J Physiol 1990;29:H1365–H1373.
Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monomine and histamine synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation 1999;99:411–419.
Hoffman BF, Cranefield PF. The Atrium. In Electrophysiology of the Heart. New York: McGraw-Hill Book Company. 1960, Chap. 3.
Cranefield PF, Aronson RS. Delayed afterdepolarizations and triggered activity in cardiac preparations not exposed to digitalis. In Cardiac Arrhythmias: The role of triggered activity and other mechanisms. Mt Kisco, New York: Futura Publishing Company. 1988, Chapt. VII.
Patterson E, Po S, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624–631.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scherlag, B.J., Nakagawa, H., Jackman, W.M. et al. Electrical Stimulation to Identify Neural Elements on the Heart: Their Role in Atrial Fibrillation. J Interv Card Electrophysiol 13 (Suppl 1), 37–42 (2005). https://doi.org/10.1007/s10840-005-2492-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10840-005-2492-2