Abstract
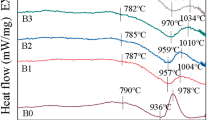
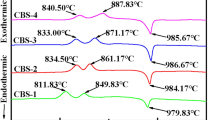
The Ba2TiSi2O8 glass-ceramics were prepared by sol–gel process using barium acetate, titanium butoxide, tetraethoxyorthosilicate and boric acid as raw materials. Because of the existence of glass and sintering additive, the Ba2TiSi2O8 glass-ceramics can be sintered at 880 °C in air. The structure of sintered sample was characterized by means of XRD and SEM. XRD patterns showed that fresnoite Ba2TiSi2O8 is the dominant crystalline phase in the sintered samples. SEM images indicated that the shape of Ba2TiSi2O8 grains varied with the molar ratio of ([Ba(Ac)2/Ti(OBun)4/Si(OEt)4]). With an increase of Si(OEt)4 content, the length/diameter ratio of Ba2TiSi2O8 grains decreases. The Ba2TiSi2O8 glass-ceramics studied in this work have ɛr in the range of 6–12 (100 MHz) and demonstrate very low dielectric losses (tanδ <2 × 10−3, 100 MHz). The experimental results suggested that the Ba2TiSi2O8 glass-ceramics could be used as dielectric materials for low temperature co-fired ceramics (LTCC) process.






Similar content being viewed by others
References
J.T. Alfons, M.C. Stinton, R.A. Matthews, A. Papst, Am. Mineral. 50, 314 (1965)
J.P. Zhang, B.I. Lee, R.W. Schwarz, J. Appl. Phys. 85, 8343 (1999)
R. Keding, C. Rüssel, J. Non-cryst. Solids. 278, 7 (2000)
A.A. Cabral, V.M. Fokin, E.D. Zanotto, C.R. Chinaglia, J. Non-cryst. Solids. 330, 174 (2003)
M.K. Zhu, Y.H. Yang, X.H. Li, B. Wang, H. wang, H. Yan, Microelectron. Eng. 66, 745 (2003)
R. Keding, C. Rüssel, J. Non-cryst. Solids. 219, 136 (1997)
J.D. Mackenzie, J. Non-cryst. Solids. 48, 1 (1982)
J. Phalippou, M. Prassas, J. Zarzycki, J. Non-cryst. Solids. 48, 17 (1982)
M.G. Ferrira da Silva, M.A. Valente, J. Non-cryst. Solids. 232–234, 409 (1998)
Y. Hu, H.T. Tsai, J. Non-cryst. Solids. 286, 51 (1998)
M. Zelner, H. Minti, R. Reisfeld, J. Sol–Gel Sci. Technol. 20, 153 (2001)
X.L. Duan, D.R. Yuan, D. Xu, et al., Mater. Res. Bull. 38, 705 (2003)
Acknowledgement
This work was supported by the Ministry of Sciences and Technology of China through 863-project under grant 2003AA32G030 and 973-project under grants of 2002CB61306 and 2001CB6104, and National Science Foundation of China under grants of 50425204, 50272032 and 90401012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, J., Zhou, J., Cui, X. et al. Synthesis and dielectric properties of Ba2TiSi2O8 glass-ceramics from the sol–gel process. J Electroceram 21, 565–568 (2008). https://doi.org/10.1007/s10832-007-9243-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-007-9243-y