Abstract
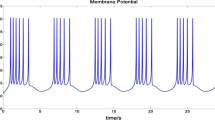
The goal of this study was to create a realistic and quantitative simulation of vasopressin (AVP) secretion under iso-osmotic and short-term challenged plasma osmolality. The relationship between AVP concentration ([AVP]) and plasma osmolality was computed using a sophisticated and integrated model that chronologically simulates (1) the overall firing rate of the hypothalamus’ magnocellular neuronal (MCN) population, (2) the propagation of the spike activity down the axons, (3) the fatigue and facilitation mechanisms of AVP release at the axon terminals and (4) the [AVP] pharmacodynamics based on the trains of AVP release. This global simulation predicted that the differential MCN sensitivity to dynorphin would be the most critical mechanism underlying the individual variability of MCN firing behaviors (silence, irregular, phasic and continuous firing patterns). However, at the level of the MCN population, the simulation predicted that the dynorphin factor must be combined with the distribution of the resting membrane potentials among the MCNs to obtain a realistic overall firing rate in response to a change in osmolality. Moreover, taking advantage of the integrated model, the simulation predicted that the selective removal of the frequency-dependent facilitation of AVP secretion has a major impact on the overall [AVP]-to-osmolality relationship (mean absolute change of 2.59 pg/ml); the action potential propagation failure, while critical, has a smaller quantitative impact on the overall [AVP] (0.58 pg/ml). The present integrated model (from a single MCN to a quantitative plasma [AVP]) improves our knowledge of the mechanisms underlying overall MCN firing and AVP excitation-secretion coupling.





Similar content being viewed by others
References
Bicknell, R. J. (1988). Optimizing release from peptide hormone secretory nerve terminals. Journal of Experimental Biology, 139, 51–65.
Bicknell, R. J., Brown, D., Chapman, C., Hancock, P. D., & Leng, G. (1984). Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. Journal of Physiology (London), 348, 601–613.
Bielefeldt, K., & Jackson, M. B. (1993). A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. Journal of Neurophysiology, 70, 284–298.
Bondy, C. A., Gainer, H., & Russell, J. T. (1987). Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology, 46, 258–267.
Bourque, C. W. (1998). Osmoregulation of vasopressin neurons: a synergy of intrinsic and synaptic processes. Progress in Brain Research, 119, 59–76.
Bourque, C. W., & Renaud, L. P. (1991). Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Research, 540, 349–352.
Brimble, M. J., & Dyball, R. E. (1977). Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. Journal of Physiology (London), 271, 253–271.
Brown, C. H., Ludwig, M., & Leng, G. (1998). kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. Journal of Neuroscience, 18, 9480–9488.
Brown, C. H., Ruan, M., Scott, V., Tobin, V. A., & Ludwig, M. (2008). Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Progress in Brain Research, 170, 219–228.
Cazalis, M., Dayanithi, G., & Nordmann, J. J. (1985). The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. Journal of Physiology (London), 369, 45–60.
Clayton, T. F., Murray, A. F., & Leng, G. (2010). Modeling the in vivo spike activity of phasically-firing vasopressin cells. Journal of Neuroendocrinology, 22, 1290–1300.
Czaczkes, J. W., & Kleeman, C. R. (1964). The effects of various states of hydration and the plasma concentration on the turnover of antidiuretic hormone in mammals. Journal of Clinical Investigation, 43, 1649–1658.
Dunn, F. L., Brennan, T. J., Nelson, A. E., & Robertson, G. L. (1973). The role of blood osmolality and volume in regulating vasopressin secretion in the rat. Journal of Clinical Investigation, 52, 3212–3219.
Dyball, R. E., Grossmann, R., Leng, G., & Shibuki, K. (1988). Spike propagation and conduction failure in the rat neural lobe. Journal of Physiology (London), 401, 241–256.
Hobbach, H., Hurth, S., Jost, D., & Racké, K. (1988). Effects of tetraethylammonium ions on frequency-dependent vasopressin release from the rat neurohypophysis. Journal of Physiology (London), 397, 539–554.
Iremonger, K. J., & Bains, J. S. (2007). Integration of asynchronously released quanta prolongs the postsynaptic spike window. Journal of Neuroscience, 27, 6684–6691.
Jackson, M. B., Konnerth, A., & Augustine, G. J. (1991). Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proceedings of the National Academy of Sciences of the United States of America, 88, 380–384.
Komandantov, A. O., Trayanova, N. A., & Tasker, J. G. (2007). Somato-dendritic mechanisms underlying the electrophysiological properties of hypothalamic magnocellular neuroendocrine cells: a multicompartmental model study. Journal of Computational Neuroscience, 23, 143–168.
Leng, G., & Ludwig, M. (2008). Neurotransmitters and peptides: whispered secrets and public announcements. Journal of Physiology (London), 586, 5625–5632.
Leng, G., Brown, C., Sabatier, N., & Scott, V. (2008a). Population dynamics in vasopressin cells. Neuroendocrinology, 88, 160–172.
Leng, G., Onaka, T., Caquineau, C., Sabatier, N., Tobin, V. A., & Takayanagi, Y. (2008b). Oxytocin and appetite. Progress in Brain Research, 170, 137–151.
Mason, W. T. (1983). Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proceedings of the Royal Society B: Biological Sciences, 217, 141–161.
Morris, J. F. (1976). Hormone storage in individual neurosecretory granules of the pituitary gland: a quantitative ultrastructural approach to hormone storage in the neural lobe. Journal of Endocrinology, 68, 209–224.
Nadeau, L., & Mouginot, D. (2011). New determinants of firing rates and patterns of vasopressinergic magnocellular neurons: predictions using a mathematical model of osmodetection. Journal of Computational Neuroscience, 31, 441–451.
Nadeau, L., Arbour, D., & Mouginot, D. (2010). Computational simulation of vasopressin secretion using a rat model of the water and electrolyte homeostasis. BMC Physiology, 10, 17.
Nordmann, J. J., & Stuenkel, E. L. (1986). Electrical properties of axons and neurohypophysial nerve terminals and their relationship to secretion in the rat. The Journal of Physiology (London), 380, 521–539.
Poulain, D. A., Brown, D., & Wakerley, J. (1988). Statistical analysis of patterns of electrical activity in vasopressin and oxytocin-secreting neurones. In: Pulsatility in neuroendocrine systems (ed. Leng G), 119–154. CRC Press, Boca Raton, FL, U.S.A
Richard, D., & Bourque, C. W. (1995). Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. The Journal of Physiology (London), 489, 567–577.
Roper, P., Callaway, J., & Armstrong, W. (2004). Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. Journal of Neuroscience, 24, 4818–4831.
Scott, V., Bishop, V. R., Leng, G., & Brown, C. H. (2009). Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. Journal of Physiology (London), 587, 5679–5689.
Verbalis, J. G. (2003). Disorders of body water homeostasis. Best Practice & Research Clinical Endocrinololgy & Metabolism, 17, 471–503.
Wakerley, J. B., Poulain, D. A., & Brown, D. (1978). Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Research, 148, 425–440.
Wakerley, J. B., Poulain, D. A., Dyball, R. E., & Cross, B. A. (1975). Activity of phasic neurosecretory cells during haemorrhage. Nature, 258, 82–84.
Walters, J. K., & Hatton, G. I. (1974). Supraoptic neuronal activity in rats during five days of water deprivation. Physiology & Behavior, 13, 661–667.
Weisinger, R. S., Burns, P., Eddie, L. W., & Wintour, E. M. (1993). Relaxin alters the plasm a osmolality-arginine vasopressin relationship in the rat. Journal of Endocrinology, 137, 505–510.
Wilson, K. C., Weitzman, R. E., & Fisher, D. A. (1978). Arginine vasopressin metabolism in dogs. II. Modeling and system analysis. American Journal of Physiology, 235, E598–605.
Acknowledgement
This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes for Health Research (CIHR; MOP-178002). LN received a scholarship from the NSERC (ESD3-334440-2006).
The authors would like to thank Drs. Colin Brown (University of Otago, Dunedin, New Zealand) and Charles Bourque (McGill University, Montreal, Canada) for their valuable comments and suggestions on the preliminary results of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Bard Ermentrout
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 45 kb)
Online Resource 2
(PDF 163 kb)
Online Resource 3
(PDF 156 kb)
Online Resource 4
(PDF 240 kb)
Online Resource 5
(PDF 52 kb)
Online Resource 6
(PDF 76 kb)
Rights and permissions
About this article
Cite this article
Nadeau, L., Mouginot, D. Quantitative prediction of vasopressin secretion using a computational population model of rat magnocellular neurons. J Comput Neurosci 33, 533–545 (2012). https://doi.org/10.1007/s10827-012-0399-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-012-0399-3