Abstract
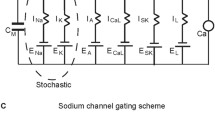
Mid-brain dopaminergic (DA) neurons display two functionally distinct modes of electrical activity: low- and high-frequency firing. The high-frequency firing is linked to important behavioral events in vivo. However, it cannot be elicited by standard manipulations in vitro. We had suggested a two-compartmental model of the DA cell that united data on firing frequencies under different experimental conditions. We now analyze dynamics of this model. The analysis was possible due to introduction of timescale separation among variables. We formulate the requirements for low and high frequencies. We found that the modulation of the SK current gating controls the frequency rise under applied depolarization. This provides a new mechanism that limits the frequency in the control conditions and allows high-frequency responses to depolarization if the SK current gating is downregulated. The mechanism is based on changing Ca2 + balance and can also be achieved by direct modulation of the balance. Interestingly, such changes do not affect the high-frequency oscillations under NMDA. Therefore, altering Ca2 + balance allows combining the high-frequency response to NMDA activation with the inability of other treatments to effectively elevate the frequency. We conclude that manipulations affecting Ca2 + balance are most effective in controlling the frequency range. This modeling prediction gives a clue to the mechanism of the high-frequency firing in the DA neuron in vivo and in vitro.









Similar content being viewed by others
References
Carlson, N. (1999). Foundations of physiological psychology (4th ed.). MA: Allyn and Bacon.
Chan, C., Guzman, J., Ilijic, E., Mercer, J. R. C., Tkatch, T., Meredith, G., et al. (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature, 447(7148), 1081–1086.
Deister, C. A., Teagarden, M. A., Wilson, C. J., & Paladini, C. A. (2009). An intrinsic neural oscillator underlies dopaminergic neuron bursting. Journal of Neuroscience, 29(50), 15888–15897.
Ji, H., Hougaard, C., Herrik, K., Straebek, D., Christophersen, P., & Shepard, P. D. (2009). Tuning the excitability of midbrain dopamine neurons by modulating the Ca2 + sensitivity of sk channels. European Journal of Neuroscience, 29(9), 1883–1895.
Johnson, S., & Wu, Y. (2004). Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Research, 1019, 293–296.
Kang, Y., & Kitai, S. (1993). A whole cell patch-clamp study on the pacemaker potential in dopaminergic neurons of rat substantia nigra compacta. Neuroscience Research, 18, 209–221.
Kuznetsov, A., Kopell, N., & Wilson, C. (2006). Transient high-frequency firing in a coupled-oscillator model of the mesencephalic dopaminergic neuron. Journal of Neurophysiology, 95, 932–937.
Lobb, C. J., Wilson, C. J., & Paladini, C. A. (2010). A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. Journal of Neurophysiology. May 5, E-pub ahead of print.
Morikawa, H., Khodakhah, K., & Williams, J. (2003). Two intracellular pathways medicate metabotropic glutamate receptor-induced Ca2 + mobilization in dopamine neurons. Journal of Neuroscience, 23, 149–157.
Nedergaard, S., Flatman, J., & Engberg, I. (1993). Nifedipine- and omega-conotoxin-sensitive Ca2 + conductances in guinea-pig substantia nigra pars compacta neurones. Journal of Physiology (London), 466, 727–747.
Overton, P., & Clark, D. (1997). Burst firing in midbrain dopaminergic neurons. Brain Research Reviews, 25, 312–334.
Ping, H., & Shepard, P. (1996). Apamin-sensitive Ca2 + -activated k+ channels regulate pacemaker activity in nigral neurons. Neuroreport, 7, 809–814.
Richards, C., Shiroyama, T., & Kitai, S. (1997). Electrophysiological and immunocytochemical characteristics of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience, 80, 545–557.
Schultz, W. (2002). Getting formal with dopamine and reward. Neuron, 36, 241–263.
Shepar, P., & Bunney, B. (1991). Repetitive firing properties of putative dopamine-containing neurons in vitro: Regulation by an apamin-sensitive Ca(2 +)-activated k+ conductance. Experimental Brain Research, 86, 141–150.
Strange, P. (2001). Antipsychotic drugs: Importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacological Review, 53, 119–133.
Strogatz, S. (1997). Nonlinear dynamics and chaos (2nd ed.). MA: Addison-Wesley.
Surmeier, D., Mercer, J., & Chan, C. (2005). Autonomous pacemakers in the basal ganglia: Who needs excitatory synapses anyway? Current Opinion in Neurobiology, 15, 312–318.
Wise, R. (2004). Dopamine, learning and motivation. Nature Reviews. Neuroscience, 5, 483–494.
Acknowledgements
The work was supported by the National Science Foundation grant DMS-0817717. AK thanks Prof. R. Perez for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Terman
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Ha, J., Kuznetsov, A. Frequency switching in a two-compartmental model of the dopaminergic neuron. J Comput Neurosci 30, 241–254 (2011). https://doi.org/10.1007/s10827-010-0251-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0251-6