Abstract
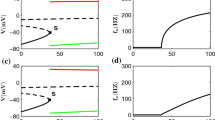
Neurons in vivo must process sensory information in the presence of significant noise. It is thus plausible to assume that neural systems have developed mechanisms to reduce this noise. Theoretical studies have shown that threshold fatigue (i.e. cumulative increases in the threshold during repetitive firing) could lead to noise reduction at certain frequencies bands and thus improved signal transmission as well as noise increases and decreased signal transmission at other frequencies: a phenomenon called noise shaping. There is, however, no experimental evidence that threshold fatigue actually occurs and, if so, that it will actually lead to noise shaping. We analyzed action potential threshold variability in intracellular recordings in vivo from pyramidal neurons in weakly electric fish and found experimental evidence for threshold fatigue: an increase in instantaneous firing rate was on average accompanied by an increase in action potential threshold. We show that, with a minor modification, the standard Hodgkin–Huxley model can reproduce this phenomenon. We next compared the performance of models with and without threshold fatigue. Our results show that threshold fatigue will lead to a more regular spike train as well as robustness to intrinsic noise via noise shaping. We finally show that the increased/reduced noise levels due to threshold fatigue correspond to decreased/increased information transmission at different frequencies.








Similar content being viewed by others
References
Aizenman, C. D., & Linden, D. J. (1999). Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. Journal of Neurophysiology, 82, 1697–1709.
Azouz, R., & Gray, C. M. (1999). Cellular mechanisms contributing to response variability of cortical neurons in vivo. Journal of Neuroscience, 19, 2209–2223.
Azouz, R., & Gray, C. M. (2000). Dynamic Spike Threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97, 8110–8115.
Barlow, H. B., & Levick, W. R. (1969a). Changes in the maintained discharge with adaptation level in the cat retina. Journal of Physiology (London), 202, 699–718.
Barlow, H. B., & Levick, W. R. (1969b). Three factors limiting the reliable detection of light by the retinal ganglion cells of the cat. Journal of Physiology (London), 200, 1–24.
Bastian, J. (1981). Electrolocation I. How the electroreceptors of Apteronotus albifrons code for moving objects and other electrical stimuli. Journal of Comparative Physiology A, 144, 465–479.
Bastian, J., & Nguyenkim, J. (2001). Dendritic Modulation of Burst-like firing in sensory neurons. Journal of Neurophysiology, 85, 10–22.
Bastian, J., Chacron. M. J., & Maler, L. (2002). Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. Journal of Neuroscience, 22, 4577–4590.
Bastian, J., Chacron, M. J., & Maler, L. (2004). Plastic and non-plastic cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron, 41, 767–779.
Borst, A., & Haag, J. (2001). Effects of mean firing on neural information rate. Journal of Computational Neuroscience, 10, 213–221.
Bryant, H. L., & Segundo, J. P. (1976). Spike initiation by transmembrane current: a white-noise analysis. Journal of Physiology, 260, 279–314.
Burns, B. D., & Webb, A. C. (1970). Spread of responses in the cerebral cortex to meaningful stimuli. Nature, 225, 469–470.
Chacron, M. J. (2006). Nonlinear information processing in a model sensory system. Journal of Neurophysiology, 95, 2933–2946.
Chacron, M.J., Longtin, A., St-Hilaire, M., & Maler, L. (2000). Suprathreshold stochastic firing dynamics with memory in P-type electroreceptors. Physical Review Letters, 85, 1576–1579.
Chacron, M. J., Longtin, A., & Maler, L. (2001a). Simple models of bursting and non-bursting electroreceptors. Neurocomputing, 38, 129–139.
Chacron, M. J., Longtin, A., & Maler, L. (2001b). Negative interspike interval correlations increase the neuronal capacity for encoding time-varying stimuli. Journal of Neuroscience, 21, 5328–5343.
Chacron, M. J., Pakdaman, K., & Longtin, A. (2003a). Interspike interval correlations, memory, adaptation, and refractoriness in a leaky integrate-and-fire model with threshold fatigue. Neural Computation, 15, 253–278.
Chacron, M. J., Doiron, B., Maler, L., Longtin, A., & Bastian, J. (2003b). Non-classical receptive field mediates switch in a sensory neuron’s frequency tuning. Nature, 423, 77–81.
Chacron, M. J., Lindner, B., & Longtin, A. (2004a). Noise shaping by interval correlations increases information transfer. Physical Review Letters, 92, 080601.1–080601.4.
Chacron, M. J., Lindner, B., & Longtin, A. (2004b). ISI correlations and information transfer. Fluctuations and Noise Letters, 4, L195–L205.
Chacron, M. J., Longtin, A., & Maler, L. (2004c). To burst or not to burst? Journal of Computational Neuroscience, 17, 127–136.
Chacron, M. J., Maler, L., & Bastian, J. (2005a). Feedback and feedforward control of frequency tuning to naturalistic stimuli. Journal of Neuroscience, 25, 5521–5532.
Chacron, M. J., Maler, L., & Bastian, J. (2005b). Electroreceptor neuron dynamics shape information transmission. Nature Neuroscience, 8, 673–678.
Cover, T., & Thomas, J. (1991). Elements of information theory. New York: Wiley.
Cox, D. R., & Lewis, P. A. W. (1966). The statistical analysis of series of events. London: Methuen.
Doiron, B., Laing, C., Longtin, A., & Maler, L. (2002). Ghostbursting: a novel neuronal burst mechanism. Journal of Computational Neuroscience, 12, 5–25.
Doiron, B., Chacron, M. J., Maler, L., Longtin, A., & Bastian, J. (2003). Inhibitory feedback required for network oscillatory responses to communication but not prey stimuli. Nature, 421, 539–543.
Fernandez, F. R., Mehaffey, W. H., & Turner, R. W. (2005). Dendritic Na+ current inactivation can increase cell excitability by delaying a somatic depolarizing afterpotential. Journal of Neurophysiology, 94, 3836–3848.
Gabbiani, F., & Koch, C. (1996). Coding of time-varying signals in spike trains of integrate-and-fire neurons with random threshold. Neural Computation, 8, 44–66.
Gabbiani, F., Metzner, W., Wessel, R., & Koch, C. (1996). From stimulus encoding to feature extraction in weakly electric fish. Nature, 384, 564–567.
Geisler, C. D., & Goldberg, J. M. (1966). A stochastic model of the repetitive activity of neurons. Biophysical Journal, 6, 53–69.
Gestri, G., Masterbroek, H. A. K., & Zaagman, W. H. (1980). Stochastic constancy, variability and adaptation of spike generation: performance of a giant neuron in the visual system of the fly. Biological Cybernetics, 38, 31–40.
Goldberg, J. M. (2000). Afferent diversity and the organisation of central vestibular pathways. Experimental Brain Research, 130, 277–297.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology London, 117, 500–544.
Holden, A. V. (1976). Models of the stochastic activity of neurons. Berlin: Springer.
Jaeger, D., & Bauer, J. M. (1994). Prolonged responses in rat cerebellar Purkinje cells following activation of the granule cell layer: an intracellular in vitro and in vivo investigation. Experimental Brain Research, 100, 200–214.
Jolivet, R., Rauch, A., Luscher, H. R., & Gerstner, W. (2006). Predicting spike timing of neocortical pyramidal neurons by simple threshold models. Journal of Computational Neuroscience, 21, 35–49.
Koch, C. (1999). Biophysics of computation. New York: Oxford University Press.
Köppl, C. (1997). Frequency tuning and spontaneous activity in the auditory nerve and Cochlear Nucleus Magnocellularis of the Barn Owl Tyto alba. Journal of Neurophysiology, 77, 364–377.
Lebedev, M. A., & Nelson, R. J. (1996). High-frequency vibratory sensitive neurons in monkey primate somatosensory cortex: entrained and nonentrained responses to vibration during the performance of vibratory-cued hand movements. Experimental Brain Research, 111, 313–325.
Lemon, N., & Turner, R. W. (2000). Conditional spike backpropagation generates burst discharge in a sensory neuron. Journal of Neurophysiology, 84, 1519–1530.
Lindner, B., Chacron, M. J., & Longtin, A. (2005). Integrate-and-fire neurons with threshold noise: A tractable model of how interspike interval correlations affect neuronal signal transmission. Physical Review E, 72, 021911.
Liu, Y. H., & Wang, X. J. (2001). Spike Frequency adaptation of a generalized leaky integrate-and-fire neuron. Journal of Computational Neuroscience, 10, 25–45.
Mainen, Z. F., & Sejnowski, T. J. (1995). Reliability of spike timing in neocortical neurons. Science, 268, 1503–1506.
Manwani, A., & Koch, C. (1999). Detecting and estimating signals in noisy cable structure, I: neuronal noise sources. Neural Computation, 11, 1797–1829.
Mar, D. J., Chow, C. C., Gerstner, W., Adams, R. W., & Collins, J. J. (1999). Noise Shaping in populations of coupled model neurons. Proceedings of the National Academy of Sciences, 96, 10450–10455.
Metzner, W., Koch, C., Wessel, R., & Gabbiani, F. (1998). Feature extraction by burst-like spike patterns in multiple sensory maps. Journal of Neuroscience, 18, 2283–2300.
Mickus, T., Jung, H. Y., & Spruston, N. (1999). Properties of slow cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophysical Journal, 76, 846–860.
Norsworthy, S. R., Schreier, R., & Temes, G. C. (Eds.) (1997). Delta-sigma data converters. Piscataway, NJ: IEEE Press.
Oswald, A. M. M., Chacron, M. J., Doiron, B., Bastian, J., & Maler, L. (2004). Parallel processing of sensory input by bursts and isolated spikes. Journal of Neuroscience, 24, 4351–4362.
Reinagel, P., & Reid, R. C. (2000). Temporal coding of visual information in the thalamus. Journal of Neuroscience, 20, 5392–5400.
Rieke, F., Warland, D., de Ruyter van Steveninck, R. R., & Bialek, W. (1996). Spikes: Exploring the neural code. Cambridge, MA: MIT.
Rudolph, M., & Destexhe, A. (2003). The discharge variability of neocortical neurons during high-conductance states. Neuroscience, 119, 855–873.
Sadeghi, S. G., Chacron, M. J., Taylor, M. C., & Cullen, K. E. (2007). Neural variability, detection thresholds, and information transmission in the vestibular system. Journal of Neuroscience, 27, 771–781.
Sah, P. (1996). Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends in Neurosciences, 19, 150–154.
Shannon, C. E. (1948). The mathematical theory of communication. Bell Systems Technical Journal, 27, 379–423, 623–656.
Sherman, S. M. (2001). Tonic and burst firing: dual modes of thalamocortical relay. Trends in Neurosciences, 24, 122–126.
Shin, J. (1993). Novel neural circuits based on stochastic pulse coding noise feedback pulse coding. International Journal of Electronics, 74, 359–368.
Shin, J. (2001). Adaptation in spiking neurons based on the noise shaping neural coding hypothesis. Neural Networks, 14, 907–919.
Shinomoto, S., Sakai, Y., & Funahashi, S. (1999). The Ornstein–Uhlenbeck process does not reproduce spiking statistics of neurons in prefrontal cortex. Neural Computation, 11, 935–951.
Softky, W. R., & Koch, C. (1993). The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. Journal of Neuroscience, 13, 334–350.
Stein, R. B., Gossen, E. R., & Jones, K. E. (2005). Neuronal variability: noise or part of the signal. Nature Reviews Neuroscience, 6, 4766–4778.
Steriade, M. (1978). Cortical long-axoned cells and putative interneurons during the sleep-waking cycle. Behavioural Brain Research, 3, 465–514.
Teich, M. C. (1992). Fractal neuronal firing patterns. In: T. McKenna, J. Davis, & S. F. Zornetzer (Eds) Single neuron computation (pp. 589–622). San Diego: Academic Press.
Teich, M. C., & Khanna, S. M. (1985). Pulse-number distributions for the neural spike train in the cat’s auditory nerve. Journal of the Accoustical Society of America 77, 1110–1128.
Treves, A. (1996). Mean-field analysis of neuronal spike dynamics. Network: Computation in Neural Systems, 4, 259–284.
Wang, X. J. (1998). Calcium coding and adaptive temporal computation in cortical pyramidal neurons. Journal of Neurophysiology, 79, 1549–1566.
Wiesenfeld, K., & Satija, I. (1987). Noise tolerance of frequency-locked dynamics. Physical Review B, 36, 2483–2492.
Yacomotti, A. M., Eguia, M. C., Aliaga, J., Martinez, O. E., & Mindlin, G. B. (1999). Interspike time distribution in noise driven excitable systems. Physical Review Letters, 83, 292–295.
Acknowledgement
We thank J. Benda for useful discussions. This research was supported by CIHR (M.J.C., A.L.) and NSERC (A.L.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Golomb
Rights and permissions
About this article
Cite this article
Chacron, M.J., Lindner, B. & Longtin, A. Threshold fatigue and information transfer. J Comput Neurosci 23, 301–311 (2007). https://doi.org/10.1007/s10827-007-0033-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0033-y