Abstract
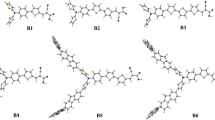
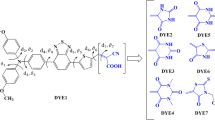
Three organic electron donors (T1, T2 and T3) were creatively synthesized by researchers, based on their extraordinary characteristics under the experimental conditions. The three original donors were selected and designed to substitute the primary donors of two dye molecules of different types LJ1 (D–π–A) and WS4 (D–A–π–A). In the subsequent theoretical investigations, density functional theory (DFT) and time-dependent DFT (TD-DFT) were employed to explore and simulate the fundamental parameters for these six potential sensitizers in dye-sensitized solar cells (DSSCs). For the essential parameters in evaluating the energy conversion process, such as oscillator strength (f) and light harvesting efficiency (LHE), the driving force of electron injection (ΔGinject) vertical dipole moments (μnormal) and driving force of regeneration (ΔGregen) were simulated. In addition, energy levels and the orbital compositions, absorption spectra, natural bond orbital (NBO) analysis from S0 to S1, distance between hole and electron centroid (DCT), average hole-electron distance (Δr) upon photoexcitation, overlap distance of hole and electron (S), transferred charge (ΔQ) for intramolecular charge transfer (ICT) processing, excited lifetime and electron injection lifetime of the six chromophores were systematically computed. Because of the different configurations formed in the donor motif, the correlative properties of the six newly designed sensitizers compared with prototype sensitizers of LJ1 and WS4 were altered and promoted significantly.




















Similar content being viewed by others
References
Grätzel, M.: Photoelectrochemical cells. Nature 414, 338–344 (2001). https://doi.org/10.1038/35104607
O’Regan, B., Gratzel, M.: A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991). https://doi.org/10.1038/353737a0
Liu, W.-H., Wu, I.-C., Lai, C.-H., Lai, C.-H., Chou, P.-T., Li, Y.-T., Chen, C.-L., Hsu, Y.-Y., Chi, Y.: Simple organic molecules bearing a 3,4-ethylenedioxythiophene linker for efficient dye-sensitized solar cells. Chem. Commun. 81, 5152 (2008). https://doi.org/10.1039/b808535h
Zhu, W., Wu, Y., Wang, S., Li, W., Li, X., Chen, J., Wang, Z.S., Tian, H.: Organic D–A–π–A solar cell sensitizers with improved stability and spectral response. Adv. Funct. Mater. 21, 756–763 (2011). https://doi.org/10.1002/adfm.201001801
Luo, C., Bi, W., Deng, S., Zhang, J., Chen, S., Li, B., Liu, Q., Peng, H., Chu, J.: Indolo[3,2,1-jk]carbazole derivatives-sensitized solar cells: effect of π-bridges on the performance of cells. J. Phys. Chem. C 118, 14211–14217 (2014). https://doi.org/10.1021/jp503455m
Ning, Z., Zhang, Q., Wu, W., Pei, H., Liu, B., Tian, H.: Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J. Org. Chem. 73, 3791–3797 (2008). https://doi.org/10.1021/jo800159t
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 98, 5648–5652 (1993). https://doi.org/10.1063/1.464913
Hariharan, P.C., Pople, J.A.: The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta. 28, 213–222 (1973). https://doi.org/10.1007/BF00533485
Li, Y., Pullerits, T., Zhao, M., Sun, M.: Theoretical characterization of the PC 60 BM:PDDTT model for an organic solar cell. J. Phys. Chem. C 115, 21865–21873 (2011). https://doi.org/10.1021/jp2040696
Capodilupo, A.L., Fabiano, E., De Marco, L., Ciccarella, G., Gigli, G., Martinelli, C., Cardone, A.: Multiwfn. J. Org. Chem. 81, 3235–3245 (2016)
Wadt, W.R., Hay, P.J.: Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys. 82, 284–298 (1985). https://doi.org/10.1063/1.448800
Li, X., Zhang, X., Hua, J., Tian, H.: Molecular engineering of organic sensitizers with o, p-dialkoxyphenyl-based bulky donors for highly efficient dye-sensitized solar cells. Mol Syst Des Eng. 2, 98–122 (2017). https://doi.org/10.1039/C7ME00002B
Ning, Z., Fu, Y., Tian, H.: Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ. Sci. 3, 1170 (2010). https://doi.org/10.1039/c003841e
Zhang, C.-R., Li, X.-Y., Shen, Y.-L., Wu, Y.-Z., Liu, Z.-J., Chen, H.-S.: Molecular docking toward panchromatic dye sensitizers for solar cells based upon tetraazulenylporphyrin and tetraanthracenylporphyrin. J. Phys. Chem. A 121, 2655–2664 (2017). https://doi.org/10.1021/acs.jpca.6b12979
Yu, G., Yang, Y., Cao, Y., Pei, Q., Zhang, C., Heeger, A.J.: Measurement of the energy gap in semiconducting polymers using the light-emitting electrochemical cell. Chem. Phys. Lett. 259, 465–468 (1996). https://doi.org/10.1016/0009-2614(96)00768-3
Capodilupo, A.L., Fabiano, E., De Marco, L., Ciccarella, G., Gigli, G., Martinelli, C., Cardone, A.: Density functional theory characterization and design of high-performance diarylamine-fluorenedyes with different π spacers for dye-sensitized solar cells. J. Org. Chem. 81, 3235–3245 (2016). https://doi.org/10.1039/C1JM13028E
Grätzel, M.: Recent advances ins sensitized mesoscopic solar cells. Acc. Chem. Res. 42, 1788–1798 (2009). https://doi.org/10.1021/ar900141y
Zhang, J., Li, H.-B., Zhang, J.-Z., Wu, Y., Geng, Y., Fu, Q., Su, Z.-M.: A promising anchor group for efficient organic dye sensitized solar cells with iodine-free redox shuttles: a theoretical evaluation. J Mater Chem A. 1, 14000 (2013). https://doi.org/10.1039/c3ta12311a
Ardo, S., Meyer, G.J.: Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO 2 semiconductor surfaces. Chem. Soc. Rev. 38, 115–164 (2009). https://doi.org/10.1039/B804321N
Hasselman, G.M., Watson, D.F., Stromberg, J.R., Bocian, D.F., Holten, D., Lindsey, J.S., Meyer, G.J.: Theoretical solar-to-electrical energy-conversion efficiencies of perylene–porphyrin light-harvesting arrays †. J Phys Chem B. 110, 25430–25440 (2006). https://doi.org/10.1021/jp064547x
Capodilupo, A.L., Fabiano, E., De Marco, L., Ciccarella, G., Gigli, G., Martinelli, C., Cardone, A.: Efficient improvements in the performance of Ru(II) π-expanded terpyridyl dyes in dye-sensitized solar cells: a theoretical study. J. Org. Chem. 81, 3235–3245 (2016). https://doi.org/10.1016/j.jphotochem.2015.01.004
Capodilupo, A.L., Fabiano, E., De Marco, L., Ciccarella, G., Gigli, G., Martinelli, C., Cardone, A.: Theoretical investigation of new thiazolothiazole-based D–π–A organic dyes for efficient dye-sensitized solar cell. J. Org. Chem. 81, 3235–3245 (2016). https://doi.org/10.1016/j.saa.2014.01.052
Zhang, J.Z., Zhang, J., Li, H.B., Wu, Y., Xu, H.L., Zhang, M., Geng, Y., Su, Z.M.: Modulation on charge recombination and light harvesting toward high-performance benzothiadiazole-based sensitizers in dye-sensitized solar cells: a theoretical investigation. J. Power Sources 267, 300–308 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.085
Zhang, J.-Z., Li, H.-B., Geng, Y., Wen, S.-Z., Zhong, R.-L., Wu, Y., Fu, Q., Su, Z.-M.: Modification on C219 by coumarin donor toward efficient sensitizer for dye sensitized solar cells: a theoretical study. Dye Pigment. 99, 127–135 (2013). https://doi.org/10.1016/j.dyepig.2013.04.026
Daeneke, T., Mozer, A.J., Uemura, Y., Makuta, S., Fekete, M., Tachibana, Y., Koumura, N., Bach, U., Spiccia, L.: Dye regeneration kinetics in dye-sensitized solar cells. J. Am. Chem. Soc. 134, 16925–16928 (2012). https://doi.org/10.1021/ja3054578
Capodilupo, A.L., Fabiano, E., De Marco, L., Ciccarella, G., Gigli, G., Martinelli, C., Cardone, A.: A qualitative index of spatial extent in charge-transfer excitations. J. Org. Chem. 81, 3235–3245 (2016). https://doi.org/10.1021/ct200308m
Guido, C.A., Cortona, P., Mennucci, B., Adamo, C.: On the metric of charge transfer molecular excitations: a simple chemical descriptor. J. Chem. Theory Comput. 9, 3118–3126 (2013). https://doi.org/10.1021/ct400337e
Yang, L.N., Sun, Z.Z., Chen, S.L., Li, Z.S.: The effects of various anchoring groups on optical and electronic properties of dyes in dye-sensitized solar cells. Dye Pigment. 99, 29–35 (2013). https://doi.org/10.1016/j.dyepig.2013.04.015
Chen, S.-L., Yang, L.-N., Li, Z.-S.: How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J. Power Sources 223, 86–93 (2013). https://doi.org/10.1016/j.jpowsour.2012.09.053
Yang, L.N., Zhou, H.Y., Sun, P.P., Chen, S.L., Li, Z.S.: A promising candidate with D–A–A–A architecture as an efficient sensitizer for dye-densitized solar cells. ChemPhysChem 16, 601–606 (2015). https://doi.org/10.1002/cphc.201402745
Yu, P., Zhang, F., Li, M., He, R.: Influence of position of auxiliary acceptor in D–A–π–A photosensitizes on photovoltaic performances of dye-sensitized solar cells. J. Mater. Sci. 50, 7333–7342 (2015). https://doi.org/10.1007/s10853-015-9290-8
Muscat, J.P., Newns, D.M.: Chemisorption on metals. Prog. Surf. Sci. 9, 1–43 (1978). https://doi.org/10.1016/0079-6816(78)90005-9
Persson, P., Lundqvist, M.J., Ernstorfer, R., Goddard, W.A., Willig, F.: Quantum chemical calculations of the influence of anchor-cum-spacer groups on femtosecond electron transfer times in dye-sensitized semiconductor nanocrystals. J. Chem. Theory Comput. 2, 441–451 (2006). https://doi.org/10.1021/ct050141x
Gomer, R.: Chemisorption on Metals. Presented at the (1975)
Sudyoadsuk, T., Pansay, S., Morada, S., Rattanawan, R., Namuangruk, S., Kaewin, T., Jungsuttiwong, S., Promarak, V.: Synthesis and characterization of D–D–π–A–type organic dyes bearing carbazole-carbazole as a donor moiety (D–D) for efficient dye-sensitized solar cells. Eur. J. Org. Chem. 2013, 5051–5063 (2013). https://doi.org/10.1002/ejoc.201300373
Fitri, A., Benjelloun, A.T., Benzakour, M., McHarfi, M., Hamidi, M., Bouachrine, M.: Theoretical investigation of new thiazolothiazole-based D–π–A organic dyes for efficient dye-sensitized solar cell. Spectrochim Acta: A Mol Biomol Spectrosc. 124, 646–654 (2014). https://doi.org/10.1016/j.saa.2014.01.052
Lu, Y.H., Liu, R.R., Zhu, K.L., Song, Y.L., Geng, Z.Y.: Theoretical study on the application of double-donor branched organic dyes in dye-sensitized solar cells. Mater. Chem. Phys. 181, 284–294 (2016). https://doi.org/10.1016/j.matchemphys.2016.06.060
Li, Y., Li, Y., Song, P., Ma, F., Liang, J., Sun, M.: Screening and design of high-performance indoline-based dyes for DSSCs. RSC Adv. 7, 20520–20536 (2017). https://doi.org/10.1039/C6RA28396A
Tan, C.J., Yang, C.S., Sheng, Y.C., Amini, H.W., Tsai, H.H.G.: Spacer effects of donor-π spacer-acceptor sensitizers on photophysical properties in dye-sensitized solar cells. J. Phys. Chem. C 120, 21272–21284 (2016). https://doi.org/10.1021/acs.jpcc.6b07032
Wang, Y., Zheng, Z., Li, T., Robertson, N., Xiang, H., Wu, W., Hua, J., Zhu, W.H., Tian, H.: D–A–π–A motif quinoxaline-based sensitizers with high molar extinction coefficient for quasi-solid-state dye-sensitized solar cells. ACS Appl. Mater. Interfaces. 8, 31016–31024 (2016). https://doi.org/10.1021/acsami.6b11152
Li, M., Kou, L., Diao, L., Zhang, Q., Li, Z., Wu, Q., Lu, W., Pan, D., Wei, Z.: Theoretical study of WS-9-based organic sensitizers for unusual Vis/NIR absorption and highly efficient dye-sensitized solar cells. J. Phys. Chem. C 119, 9782–9790 (2015). https://doi.org/10.1021/acs.jpcc.5b03667
Li, S.-B., Gu, D.-M., Zhang, J., Geng, Y., Zhang, M., Su, Z.-M.: Theoretical design and characterization of high-efficiency organic dyes with different electron-withdrawing groups based on C275 toward dye-sensitized solar cells. New J. Chem. 40, 9320–9328 (2016). https://doi.org/10.1039/c6nj01731b
Cossi, M., Barone, V.: Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys. 115, 4708–4717 (2001). https://doi.org/10.1063/1.1394921
Zhang, C.R., Liu, L., Zhe, J.W., Jin, N.Z., Ma, Y., Yuan, L.H., Zhang, M.L., Wu, Y.Z., Liu, Z.J., Chen, H.S.: The role of the conjugate bridge in electronic structures and related properties of tetrahydroquinoline for dye sensitized solar cells. Int. J. Mol. Sci. 14, 5461–5481 (2013). https://doi.org/10.3390/ijms14035461
Lin, L.Y., Tsai, C.H., Wong, K.T., Huang, T.W., Hsieh, L., Liu, S.H., Lin, H.W., Wu, C.C., Chou, S.H., Chen, S.H., Tsai, A.I.: Organic dyes containing coplanar diphenyl-substituted dithienosilole core for efficient dye-sensitized solar cells. J. Org. Chem. 75, 4778–4785 (2010). https://doi.org/10.1021/jo100762t
Acknowledgments
We gratefully acknowledge computing resources support from the Supercomputing Center of Cold and Arid Region Environment and Engineering Research Institute of Chinese Academy of Sciences, Gansu Province Supercomputer Center and Institute of Functional Material Chemistry of Northeast Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Lu, X., Sheng, Y. et al. Theoretical investigations on newly designed triphenylamine-based donors applied into the D–π–A and D–A–π–A type sensitizers. J Comput Electron 17, 1816–1834 (2018). https://doi.org/10.1007/s10825-018-1246-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-018-1246-1