Abstract
Purpose
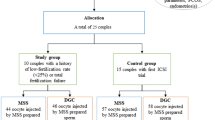
It is known that sperm preparation techniques in in vitro fertilisation (IVF) are intended to select the best-quality sperm. The aim of this study is to compare sperm the density gradient method and microfluidic chip (Fertile Plus) method in infertile patients by analysing fertilisation rates, pregnancy rates, and sperm morphology and DNA fragmentation rates posed by these two methods.
Methods
Using semen samples obtained from the patients, sperms were prepared with gradient (n = 312) and microfluidic chip methods (n = 116). Fertilisation and pregnancy rates were compared in the first time and in the recurrent IVF trial patients. In addition, the morphology and DNA fragmentation comparison of sperm samples were evaluated by Toluidine blue in situ chemical staining method.
Results
There was no statistically significant difference between fertilisation and pregnancy rates when compared with study groups in first-time IVF treatment patients. However, in recurrent IVF failure patients, there was a significant difference in fertilisation rates but no statistically significant difference was found in pregnancy rates. The microfluidic chip method significantly decreased sperm DNA fragmentation index according to density gradient method.
Conclusions
Microfluidic chip method may be recommended in patients with recurrent unsuccessful in vitro trials. The sperm DNA fragmentation test prior to the treatment will be helpful in selecting the appropriate sperm-washing method.

Similar content being viewed by others
References
Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26.
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356.
Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–12.
Tanphaichitr N, Agulnick A, Seibel M, Taymor M. Comparison of the in vitro fertilization rate by human sperm capacitated by multiple-tube swim-up and Percoll gradient centrifugation. J In Vitro Fert Embryo Transf. 1988;5(3):119–22.
Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988;9(6):367–76.
Tasoglu S, Safaee H, Zhang X, Kingsley JL, Catalano PN, Gurkan UA, et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small. 2013;9(20):3374–84.
Belva F, Bonduelle M, Roelants M, Michielsen D, Van Steirteghem A, Verheyen G, et al. Semen quality of young adult ICSI offspring: the first results. Hum Reprod. 2016;31:2811–20.
Schieve LA, Peterson HB, Meikle SF, Jeng G, Danel I, Burnett NM, et al. Live-birth rates and multiple-birth risk using in vitro fertilization. JAMA. 1999;282(19):1832–8.
Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16(10):2160–5.
Avendaño C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94:549–55.
Yetkinel S, Kilicdag EB, Aytac PC, Haydardedeoglu B, Simsek E, Cok T. Effects of the microfluidic chip technique in sperm selection for intracytoplasmic sperm injection for unexplained infertility: a prospective, randomized controlled trial. J Assist Reprod Genet. 2018;18:1375–2.
Van Der Zwalmen P, Bertin-Segal G, Geerts L, Debauche D, Schoysman R. Sperm morphology and IVF pregnancy rate: comparison between Percoll gradient centrifugation and swim-up procedures. Hum Reprod. 1991;6(4):581–8.
Prakash P, Leykin L, Chen Z, Toth T, Sayegh R, Schiff I, et al. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria). Fertil Steril. 1998;69(4):722–6.
Muratori M, Tarozzi N, Cambi M, Boni L, Iorio AL, Passaro C, et al. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: a possible new male predictive parameter of pregnancy? Medicine (Baltimore). 2016;95(20):e3624.
Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56(6):1081–4.
Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74(4):824–7.
Wang M, Sun J, Wang L, Gao X, Lu X, Wu Z, et al. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 2014;31(12):1655–63.
Ghaleno LR, Valojerdi MR, Janzamin E, Chehrazi M, Sharbatoghli M, Yazdi RS. Evaluation of conventional semen parameters, intracellular reactive oxygen species, DNA fragmentation and dysfunction of mitochondrial membrane potential after semen preparation techniques: a flow cytometric study. Arch Gynecol Obstet. 2014;289:173–80.
Jackson RE, Bormann CL, Hassun PA, Rocha AM, Motta EL, Serafini PC. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril. 2010;94:2626–30.
Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557–63.
Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33(8):1388–93.
Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0165125.
Rui BR, Angrimani D, Bicudo LC, Losano J, Nichi M, Pereira R. A fast, low-cost and efficient method for the diagnosis of sperm DNA fragmentation in several species. Reprod Domest Anim. 2018;53:171–5.
Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod. 2004;19:2277–82.
Tsarev I, Bungum M, Giwercman A, Erenpreisa J, Ebessen T, Ernst E, et al. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod. 2009;24(7):1569–74.
Pourmasumi S, Khoradmehr A, Rahiminia T, Sabeti P, Talebi A, Ghasemzadeh J, et al. Evaluation of sperm chromatin integrity using aniline blue and toluidine blue staining in infertile and normozoospermic men. J Reprod Infertil. 2019;20(2):95–101.
Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. J In Vitro Fert Embryo Transf. 2006;65(5):979–91.
Zini A, Jamal W, Cowan L, Al-Hathal N. Is sperm DNA damage associated with IVF embryo quality? A systematic review. J Assist Reprod Genet. 2011;28(5):391–7.
Funding
This study was funded by Inonu University Research Fund (2016/147).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was submitted to and has been approved by Malatya Province Clinical Research Council of Ethics with the protocol number 2017-34.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yildiz, K., Yuksel, S. Use of microfluidic sperm extraction chips as an alternative method in patients with recurrent in vitro fertilisation failure. J Assist Reprod Genet 36, 1423–1429 (2019). https://doi.org/10.1007/s10815-019-01480-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01480-3