Abstract
Purpose
The purpose of the study was to compare the morphokinetic parameters of embryos carrying balanced chromosomal translocations with those carrying unbalanced chromosomal translocations using time-lapse microscopy.
Methods
The study group included 270 embryos that underwent biopsies on day 3 for preimplantation genetic diagnosis (PGD) for chromosomal translocations in our unit between 2013 and 2015. All embryos were incubated under time-lapse microscopy and evaluated for timing of developmental events up to day 5. The timing of these events was compared between balanced and unbalanced embryos, potentially viable and nonviable variants, and maternal versus paternal inheritance of the translocation.
Results
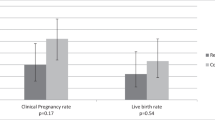
The PGD analysis found that 209 (77%) of the 270 biopsied embryos carried an unbalanced translocation. Embryos carrying unbalanced translocations, which are expected to lead to implantation failure or miscarriage, cleaved less synchronously and were delayed in time of cleavage to the 4-cell stage (t4) and in time of start of blastulation (tSB) compared with balanced embryos (P < 0.05). Furthermore, embryos carrying nonviable translocations demonstrated a significant delay at the time of pronuclei fading (tPNf) compared with those carrying potentially viable translocations (P < 0.05). Embryos whose unbalanced translocations were of maternal origin were significantly delayed in most of the morphokinetic parameters (including tPNf, t2, t3, t4, t6, t7, t8, cc2, s2, and tSB) compared with embryos carrying balanced translocations (P < 0.05).
Conclusions
Embryos carrying unbalanced chromosomal translocations mainly of maternal origin undergo delayed development and asynchronous cleavage that may lead to implantation failure or miscarriage.



Similar content being viewed by others
References
Liss J, Kiewisz J, Zabielska J, Kulwikowska P, Lukaszuk K. Application of FISH method for preimplantation genetic diagnostics of reciprocal and Robertsonian translocations. Folia Histochem Cytobiol. 2015;53:162–8.
Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94:283–9.
Chen CK, Wu D, Yu HT, Lin CY, Wang ML, Yeh HY, et al. Preimplantation genetic diagnosis by fluorescence in situ hybridization of reciprocal and Robertsonian translocations. Taiwan J Obstet Gynecol. 2014;53:48–52.
Loh SF, Wong PC, Jiang B, Yeo GH, Tan AS, Prasath EB, et al. Preimplantation genetic diagnosis of chromosome translocations by analysis of polymorphic short tandem repeats. Singap Med J. 2012;53:648–54.
Stern C, Pertile M, Norris H, Hale L, Baker HW. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum Reprod. 1999;14:2097–101.
Lledo B, Ortiz JA, Morales R, Ten J, de la Fuente PE, Garcia-Ochoa C, et al. The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Hum Reprod. 2010;25:1843–8.
Escudero T, Abdelhadi I, Sandalinas M, Munne S. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril. 2003;79(Suppl 3):1528–34.
Scriven PN, Kirby TL, Ogilvie CM. FISH for pre-implantation genetic diagnosis. J Vis Exp. 2011. https://doi.org/10.3791/2570
Colls P, Escudero T, Fischer J, Cekleniak NA, Ben-Ozer S, Meyer B, et al. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online. 2012;24:621–9.
Kirkegaard K, Ahlstrom A, Ingerslev HJ, Hardarson T. Choosing the best embryo by time lapse versus standard morphology. Fertil Steril. 2015;103:323–32.
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29:2650–60.
Wong C, Chen AA, Behr B, Shen S. Time-lapse microscopy and image analysis in basic and clinical embryo development research. Reprod BioMed Online. 2013;26:120–9.
Aparicio-Ruiz B, Basile N, Perez Albala S, Bronet F, Remohi J, Meseguer M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: retrospective study in oocyte donation. Fertil Steril. 2016;106:1379–85 e10.
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035–43.
Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–4.
Herrero J, Tejera A, Albert C, Vidal C, de los Santos MJ, Meseguer M. A time to look back: analysis of morphokinetic characteristics of human embryo development. Fertil Steril. 2013;100:1602–9 e1–4.
Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–85.
Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71.
Campbell A, Fishel S, Laegdsmand M. Aneuploidy is a key causal factor of delays in blastulation: author response to 'A cautionary note against aneuploidy risk assessment using time-lapse imaging'. Reprod BioMed Online. 2014;28:279–83.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod BioMed Online. 2013;26:477–85.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod BioMed Online. 2013;27:140–6.
Coonen E, Dumoulin JC, Ramaekers FC, Hopman AH. Optimal preparation of preimplantation embryo interphase nuclei for analysis by fluorescence in-situ hybridization. Hum Reprod. 1994;9:533–7.
Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, et al. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009;92:890–6.
Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38:52–4.
Dutta UR, Rajitha P, Pidugu VK, Dalal AB. Cytogenetic abnormalities in 1162 couples with recurrent miscarriages in southern region of India: report and review. J Assist Reprod Genet. 2011;28:145–9.
Davies S, Christopikou D, Tsorva E, Karagianni A, Handyside AH, Mastrominas M. Delayed cleavage division and prolonged transition between 2 and 4 cell stages in embryos identified as aneuploidy at 8 cell stage in array CGH. Hum Reprod. 2012;27:ii84–i6.
Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun. 2012;3:1251.
Basile N, Nogales Mdel C, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101:699–704.
Patel DV, Shah PB, Kotdawala AP, Herrero J, Rubio I, Banker MR. Morphokinetic behavior of euploid and aneuploid embryos analyzed by time-lapse in embryoscope. J Hum Reprod Sci. 2016;9:112–8.
Yang Z, Zhang J, Salem SA, Liu X, Kuang Y, Salem RD, et al. Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genet. 2014;7:38.
Chawla M, Fakih M, Shunnar A, Bayram A, Hellani A, Perumal V, et al. Morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy in a retrospective time-lapse imaging study. J Assist Reprod Genet. 2015;32:69–75.
Wharf E, Dimitrakopoulos A, Khalaf Y, Pickering S. Early embryo development is an indicator of implantation potential. Reprod BioMed Online. 2004;8:212–8.
Fancsovits P, Toth L, Takacs ZF, Murber A, Papp Z, Urbancsek J. Early pronuclear breakdown is a good indicator of embryo quality and viability. Fertil Steril. 2005;84:881–7.
Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Hum Reprod. 2003;18:1307–12.
Aguilar J, Motato Y, Escriba MJ, Ojeda M, Munoz E, Meseguer M. The human first cell cycle: impact on implantation. Reprod BioMed Online. 2014;28:475–84.
Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod BioMed Online. 2008;17:385–91.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–21.
Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly (dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–7.
Vozdova M, Oracova E, Kasikova K, Prinosilova P, Rybar R, Horinova V, et al. Balanced chromosomal translocations in men: relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J Assist Reprod Genet. 2013;30:391–405.
Dong Y, Du RC, Jiang YT, Wu J, Li LL, Liu RZ. Impact of chromosomal translocations on male infertility, semen quality, testicular volume and reproductive hormone levels. J Int Med Res. 2012;40:2274–83.
Zhang HG, Wang RX, Li LL, Sun WT, Zhang HY, Liu RZ. Male carriers of balanced reciprocal translocations in Northeast China: sperm count, reproductive performance, and genetic counseling. Genet Mol Res. 2015;14:18792–8.
Sobotka V, Vozdova M, Heracek J, Rubes J. A rare Robertsonian translocation rob(14;22) carrier with azoospermia, meiotic defects, and testicular sperm aneuploidy. Syst Biol Reprod Med. 2015;61:245–50.
Nishikawa N, Sato T, Suzumori N, Sonta S, Suzumori K. Meiotic segregation analysis in male translocation carriers by using fluorescent in situ hybridization. Int J Androl. 2008;31:60–6.
Ko DS, Cho JW, Lee HS, Kim JY, Kang IS, Yang KM, et al. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of robertsonian translocation carriers. Fertil Steril. 2013;99:1369–76.
Oliver-Bonet M, Benet J, Sun F, Navarro J, Abad C, Liehr T, et al. Meiotic studies in two human reciprocal translocations and their association with spermatogenic failure. Hum Reprod. 2005;20:683–8.
Bar-El, et al. Blastomere biopsy for PGD delays embryo compaction and blastulation: a time-lapse microscopic analysis. JARG. 2016;33:1449–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the Tel Aviv Medical Center and institutional review board approval for retrieving IVF data was obtained (748/15).
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 21.7 kb)
Supplemental Fig. 1
Comparison between the distribution of time-lapse morphokinetic parameters of embryos carrying balanced translocations with maternal and paternal origin (marked as 1) and embryos carrying unbalanced translocations with maternal origin (marked as 2). Pronuclei fading (tPNf), t2, t3, t4, t6, t7, t8 = time (h) between ICSI and pronuclei fading, two-, three-, four-, six-, seven and eight-cell stage, respectively; cc2 = length (h) of the second cell cycle; s2 = synchrony (h) in the division from three to four cells. Initiation of blastulation (tSB) is defined by time (h) between ICSI and initiation of blastulation. The mean (for tPNf, t2, t3, t4, t6, t7, t8 and tSB) and median (for cc2 and s2) values are indicated by red dots. *P < 0.05, **P < 0.01, ***P < 0.001. (PNG 35.4 kb)
Rights and permissions
About this article
Cite this article
Amir, H., Barbash-Hazan, S., Kalma, Y. et al. Time-lapse imaging reveals delayed development of embryos carrying unbalanced chromosomal translocations. J Assist Reprod Genet 36, 315–324 (2019). https://doi.org/10.1007/s10815-018-1361-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1361-8