Abstract
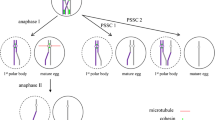
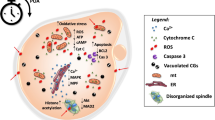
The aging-related decline in fertility is an increasingly pressing medical and economic issue in modern society where women are delaying family building. Increasingly sophisticated, costly, and often increasingly invasive, assisted reproductive clinical protocols and laboratory technologies (ART) have helped many older women achieve their reproductive goals. Current ART procedures have not been able to address the fundamental problem of oocyte aging, the increased rate of egg aneuploidy, and the decline of developmental potential of the eggs. Oocyte maturation, which is triggered by luteinizing hormone (LH) in vivo or by injection of human chorionic gonadotropin (hCG) in an in vitro fertilization (IVF) clinic, is the critical stage at which the majority of egg aneuploidies arise and when much of an egg’s developmental potential is established. Our proposed strategy focuses on improving egg quality in older women by restoring a robust oocyte maturation process. We have identified putrescine deficiency as one of the causes of poor egg quality in an aged mouse model. Putrescine is a biogenic polyamine naturally produced in peri-ovulatory ovaries. Peri-ovulatory putrescine supplementation has reduced egg aneuploidy, improved embryo quality, and reduced miscarriage rates in aged mice. In this paper, we review the literature on putrescine, its occurrence and physiology in living organisms, and its unique role in oocyte maturation. Preliminary human data demonstrates that there is a maternal aging-related deficiency in ovarian ornithine decarboxylase (ODC), the enzyme responsible for putrescine production. We argue that peri-ovulatory putrescine supplementation holds great promise as a natural and effective therapy for infertility in women of advanced maternal age, applicable in natural conception and in combination with current ART therapies.




Similar content being viewed by others
Abbreviations
- AZ:
-
Antizyme
- COC:
-
Cumulus-oocyte complex
- dcSAM:
-
Decarboxylased SAM
- DFMO:
-
D,L-α-Difluoromethylornithine
- GABA:
-
Gamma-aminobutyric acid
- GV:
-
Germinal vesicle or oocyte nucleus
- hCG:
-
Human chorionic gonadotropin
- IVM:
-
Oocyte in vitro maturation
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- ODC:
-
Ornithine decarboxylase
- ROS:
-
Reactive oxygen species
- PDE3A:
-
Phosphodiesterase 3A
- SAM:
-
S-adenosylmethionine
- SAM-DC:
-
SAM decarboxylase
- SSAT:
-
Spermidine/spermine N1 acetyltransferase
References
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee opinion no. 589. Fertil Steril. 2014;101:633–4.
Angell RR. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383–7.
Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Tejada F, et al. Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization. Endocrinology. 2005;146:666–74.
Bell MR, Belarde JA, Johnson HF, Aizenman CD. A neuroprotective role for polyamines in a Xenopus tadpole model of epilepsy. Nat Neurosci. 2011;14:505–12.
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–95.
Brown HM, Dunning KR, Sutton-McDowall M, Gilchrist RB, Thompson JG, Russell DL. Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153:R109–20.
Bruun L, Houen G. In situ detection of diamine oxidase activity using enhanced chemiluminescence. Anal Biochem. 1996;233:130–6.
Cayre M, Strambi C, Charpin P, Augier R, Strambi A. Specific requirement of putrescine for the mitogenic action of juvenile hormone on adult insect neuroblasts. Proc Natl Acad Sci U S A. 1997;94:8238–42.
Cohen SS. Pathways of polyamine metabolism in animals. In: A guide to the polyamines. Oxford: Oxford University Press; 1998. p. 208–30.
Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24:245–66.
De Vos M, Smitz J, Thompson JG, and Gilchrist RB. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31:2011–2015.
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14.
Eppig JJ. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol. 1982;89:268–72.
Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9.
Fenelon JC, Banerjee A, Lefevre P, Gratian F, Murphy BD. Polyamine-mediated effects of prolactin dictate emergence from mink obligate embryonic diapause. Biol Reprod. 2016;95:6.
Fozard JR, Part ML, Prakash NJ, Grove J, Schechter PJ, Sjoerdsma A, et al. L-ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science. 1980a;208:505–8.
Fozard JR, Prakash NJ, Grove J. Ovarian function in the rat following irreversible inhibition of L-ornithine decarboxylase. Life Sci. 1980b;27:2277–83.
Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–92.
Grant AL, Holland RE, Thomas JW, King KJ, Liesman JS. Effects of dietary amines on the small intestine in calves fed soybean protein. J Nutr. 1989;119:1034–41.
Grant AL, Thomas JW, King KJ, Liesman JS. Effects of dietary amines on small intestinal variables in neonatal pigs fed soy protein isolate. J Anim Sci. 1990;68:363–71.
Guzman L, Ortega-Hrepich C, Albuz FK, Verheyen G, Devroey P, Smitz J, et al. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–7.
Halonen T, Sivenius J, Miettinen R, Halmekyto M, Kauppinen R, Sinervirta R, et al. Elevated seizure threshold and impaired spatial learning in transgenic mice with putrescine overproduction in the brain. Eur J Neurosci. 1993;5:1233–9.
Icekson I, Kaye AM, Lieberman ME, Lamprecht SA, Lahav M, Lindner HR. Stimulation by luteinizing hormone of ornithine decarboxylase in rat ovary: preferential response by follicular tissue. J Endocrinol. 1974;63:417–8.
Iorgulescu JB, Patel SP, Louro J, Andrade CM, Sanchez AR, Pearse DD. Acute putrescine supplementation with Schwann cell implantation improves sensory and serotonergic axon growth and functional recovery in spinal cord injured rats. Neural Plast. 2015;2015:186385.
Johnson RF, Beltz TG, Thunhorst RL, Johnson AK. Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R394–403.
Kaye AM, Icekson I, Lamprecht SA, Gruss R, Tsafriri A, Lindner HR. Stimulation of ornithine decarboxylase activity by luteinizing hormone in immature and adult rat ovaries. Biochemistry. 1973;12:3072–6.
Kobayashi Y, Kupelian J, Maudsley DV. Ornithine decarboxylase stimulation in rat ovary by luteinizing hormone. Science. 1971;172:379–80.
Kong X, Wang X, Yin Y, Li X, Gao H, Bazer FW, et al. Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod. 2014;91:106.
Lefevre PL, Palin MF, Chen G, Turecki G, Murphy BD. Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology. 2011;152:1627–39.
Liang XH, Zhao ZA, Deng WB, Tian Z, Lei W, Xu X, et al. Estrogen regulates amiloride-binding protein 1 through CCAAT/enhancer-binding protein-beta in mouse uterus during embryo implantation and decidualization. Endocrinology. 2010;151:5007–16.
Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017;29:1392–1400.
Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017;29:1392–400.
Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, et al. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013;28:707–17.
Maudsley DV, Kobayashi Y. Induction of ornithine decarboxylase in rat ovary after administration of luteinizing hormone or human chorionic gonadotrophin. Biochem Pharmacol. 1974;23:2697–703.
Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105:548–59.
Metcalf BW, Bey P, Danzin C, Jung MJ, Casara P, Vevert JP. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogues. J Am Chem Soc. 1978;100:2551–3.
Moor RM, Dai Y, Lee C, Fulka J Jr. Oocyte maturation and embryonic failure. Hum Reprod Update. 1998;4:223–36.
Muller M, Cleef M, Rohn G, Bonnekoh P, Pajunen AE, Bernstein HG, et al. Ornithine decarboxylase in reversible cerebral ischemia: an immunohistochemical study. Acta Neuropathol. 1991;83:39–45.
Murakami Y, Matsufuji S, Kameji T, Hayashi S, Igarashi K, Tamura T, et al. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360:597–9.
Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11:1046–54.
Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, et al. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med. 2006;10:933–45.
Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem (Tokyo). 2006;139:81–90.
Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199–213.
Ortega-Hrepich C, Stoop D, Guzman L, Van LL, Tournaye H, Smitz J, et al. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100:1002–7.
Paschen W, Csiba L, Rohn G, Bereczki D. Polyamine metabolism in transient focal ischemia of rat brain. Brain Res. 1991;566:354–7.
Pegg AE, Casero RA Jr. Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35.
Pegg AE, McGovern KA, Wiest L. Decarboxylation of alpha-difluoromethylornithine by ornithine decarboxylase. Biochem J. 1987;241:305–7.
Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–58.
Persson L, Isaksson K, Rosengren E, Sundler F. Distribution of ornithine decarboxylase in ovaries of rat and hamster during pro-oestrus. Acta Endocrinol. 1986;113:403–9.
Quinn MC, McGregor SB, Stanton JL, Hessian PA, Gillett WR, Green DP. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18:501–8.
Raina A, Eloranta T, Kajander O. Biosynthesis and metabolism of polyamines and S-adenosylmethionine in the rat. Biochem Soc Trans. 1976;4:968–71.
Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Hoog C, Kitajima TS. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat Commun. 2015;6:7550.
Schwartz B, Hittelman A, Daneshvar L, Basu HS, Marton LJ, Feuerstein BG. A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem J. 1995;312(Pt 1):83–90.
Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. From the cover: prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–24.
Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32.
Sunkara PS, Wright DA, Nishioka K. An essential role for putrescine biosynthesis during meiotic maturation of amphibian oocytes. Dev Biol. 1981;87:351–5.
Tao Y, Liu D, Mo G, Wang H, Liu XJ. Peri-ovulatory putrescine supplementation reduces embryo resorption in older mice. Hum Reprod. 2015;30:1867–75.
Tao Y, Liu XJ. Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell. 2013;12:42–9.
Til HP, Falke HE, Prinsen MK, Willems MI. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem Toxicol. 1997;35:337–48.
Wagner J, Claverie N, Danzin C. A rapid high-performance liquid chromatographic procedure for the simultaneous determination of methionine, ethionine, S-adenosylmethionine, S-adenosylethionine, and the natural polyamines in rat tissues. Anal Biochem. 1984;140:108–16.
Washkowitz AJ, Schall C, Zhang K, Wurst W, Floss T, Mager J, et al. Mga is essential for the survival of pluripotent cells during peri-implantation development. Develop. 2015;142:31–40.
Younglai EV, Godeau F, Mester J, Baulieu EE. Increased ornithine decarboxylase activity during meiotic maturation in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1980;96:1274–81.
Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Develop. 2014;141:199–208.
Zhang M, Pickart CM, Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22:1488–96.
Zhou Y, Ma C, Karmouch J, Katbi HA, Liu XJ. Antiapoptotic role for ornithine decarboxylase during oocyte maturation. Mol Cell Biol. 2009;29:1786–95.
Funding
Supported by a research grant from March of Dimes (6-FY13-126) (to XJL), and by the Office of the Director, National Institutes of Health under Award Number P51OD011092 (to ONPRC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, Y., Tartia, A., Lawson, M. et al. Can peri-ovulatory putrescine supplementation improve egg quality in older infertile women?. J Assist Reprod Genet 36, 395–402 (2019). https://doi.org/10.1007/s10815-018-1327-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1327-x