Abstract
Purpose
Undesirable side effects of cancer treatments are common and include damage to the ovary, and depletion of the follicle reserve, which if severe enough, can lead to infertility and early menopause. Antimetabolite drugs, such as 5-fluorouracil (5-FU), are not considered to be detrimental to the ovary, but the ovotoxicity of 5-FU has not been evaluated in any detail. The purpose of this study was to evaluate the effects of 5-FU on follicle number.
Methods
In this study, adult female C57Bl6 mice (n = 4–6 animals/group) received a single dose of saline or 5-FU (150 mg/kg) and markers of ovarian damage and follicle depletion were assessed 12 h and 7 days later.
Results
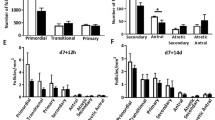
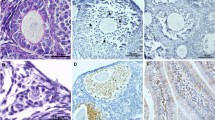
Exposure to 5-FU did not alter primordial and primary follicle numbers. Atresia of secondary and antral follicles was increased significantly 12 h after 5-FU treatment, but atresia rates returned to levels similar to that of saline treated controls at 7 days. The number of corpora lutea were reduced 7 days after exposure to 5-FU, possibly as a consequence of earlier follicular atresia.
Conclusions
These findings suggest that a single dose of 5-FU is mildly ovotoxic, but any effects on ovarian function are likely transient because the primordial follicle population is not depleted. Collectively, these data support the notion that 5-FU is unlikely to impact on the long-term fertility of women.



Similar content being viewed by others
References
Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169(1):123–31.
Meirow D, et al. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39.
Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–79.
O'Neill MT, Ni Dhonnchu T, Brannigan AE. Topic update: effects of colorectal cancer treatments on female fertility and potential methods for fertility preservation. Dis Colon Rectum. 2011;54(3):363–9.
Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015;30(12):2926–35.
Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(18):4354–9.
Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomark Prev. 2009;18(6):1695–8.
DeSantis CE, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71.
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8.
Brincker H, Mouridsen H, Andersen K. Adjuvant chemotherapy with cyclophosphamide or CMF in premenopausal women with stage II breast cancer. Breast Cancer Res Treat. 1983;3(1):91–5.
Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–29.
Hosein PJ, Rocha-Lima CM. Role of combined-modality therapy in the management of locally advanced rectal cancer. Clin Colorectal Cancer. 2008;7(6):369–75.
Cercek A, Siegel CL, Capanu M, Reidy-Lagunes D, Saltz LB. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer. 2013;12(3):163–7.
Dynes, J., The effects of chemotherapy schedules on ovarian function. 2014.
Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y, Terasawa T, et al. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–9.
Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control. 2002;9(6):466–72.
Hrushesky WJ, Vyzula R, Wood PA. Fertility maintenance and 5-fluorouracil timing within the mammalian fertility cycle. Reprod Toxicol. 1999;13(5):413–20.
Tal, R., et al., A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology, 2016: p. en20161418.
Dunlop CE, Anderson RA. Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–50.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31.
Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101(22):1543–52.
Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187(2):217–23.
Hollander GA, et al. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373(6512):350–3.
Liew SH, Vaithiyanathan K, Cook M, Bouillet P, Scott CL, Kerr JB, et al. Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol Reprod. 2014;90(4):77.
Geuna S, Herrera-Rincon C. Update on stereology for light microscopy. Cell Tissue Res. 2015;360(1):5–12.
Liew SH, Nguyen QN, Strasser A, Findlay JK, Hutt KJ. The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF. Cell Death Dis. 2017;8(8):e2971.
Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–80.
Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(3):229–63.
Howard, V. and M. Reed, Unbiased stereology: three-dimensional measurement in microscopy. 2004: Garland Science.
Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol Reprod. 2009;81(1):16–25.
Acknowledgements
We thank the Monash Micro Imaging and the Monash Histology Platform.
Funding
This work was supported by the National Health and Medical Research Council (KJH #1050130). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. The funding bodies played no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
KJH and HA designed the experiments; ML, SHL, KH and JMS performed the experiments; ML and KJH interpreted the data; ML and KJH wrote the manuscript; HA, SHL, KH and JMS critically revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Lambouras, M., Liew, S.H., Horvay, K. et al. Examination of the ovotoxicity of 5-fluorouracil in mice. J Assist Reprod Genet 35, 1053–1060 (2018). https://doi.org/10.1007/s10815-018-1169-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1169-6