Abstract
Purpose
The aim of this study was to compare the effect of the deselection of spermatozoa presenting vacuole-like structures using IMSI (intracytoplasmic morphologically selected sperm injection) with ICSI (intracytoplasmic sperm injection) by means of neonatal outcomes.
Methods
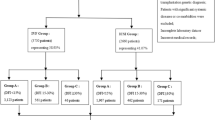
In a retrospective two-center analysis, a total of 848 successful IMSI or ICSI cycles ending with a live birth, induced abortion, or intrauterine fetal death (IUFD) were included.
Results
The IMSI and ICSI groups included 332 and 655 babies or fetuses, respectively. The parents were older in the IMSI group than in the ICSI group (mothers were 35.1 vs 32.9 years, and fathers were 39.1 vs 36.2 years). The multiple pregnancy rate was higher in the IMSI group. The mean pregnancy duration and mean birth weight were almost identical in both groups. There was no significant difference in major congenital malformations between the two groups. However, this rate was decreased in the IMSI group compared to that in the ICSI group (1.8 vs 3.2%), the difference being mainly found in singletons (1.4 vs 3.3%). Boys were more often affected than girls in both groups. The percentages of chromosomal abnormalities did not differ between the IMSI and ICSI groups (0.6 and 0.8%). The reported congenital malformations mainly affected the heart, urogenital, and musculoskeletal systems.
Conclusions
In the present study, the malformation rates observed in the IMSI and ICSI groups were not significantly different, even if slightly lower after IMSI. However, the observed difference followed the same trends observed in previous reports, indicating the possible impact of IMSI on decreasing congenital malformation occurrences. This highlights the necessity to prospectively evaluate the impact of IMSI on neonatal outcome after IVF treatment.

Similar content being viewed by others
References
David G, Bisson J, Czyglik F, Jouannet P, Gernigo N. Anomalies morphologiques du spermatozoïde humain. (1) Propositions pour un système de classification. J Gynecol Obstet Biol Reprod. 1975;4:17–36.
Kruger T, Menkveld R, Stander F, Lombard C, Van der Merwe J, van Zyl J, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45.
Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–8.
Tanaka A, Nagayoshi M, Tanaka I, Kusunoki H. Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil Steril. 2012;98:315–20.
Garolla A, Fortini D, Menegazzo M, De Toni L, Nicoletti V, Moretti A, et al. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod BioMed Online. 2008;17:610–6.
Boitrelle F, Ferfouri F, Petit JM, Segretain D, Tourain C, Bergere M, et al. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8.
Boitrelle F, Albert M, Petit JM, Ferfouri F, Wainer R, Bergere M, et al. Small human sperm vacuoles observed under high magnification are pocket-like nuclear concavities linked to chromatin condensation failure. Reprod BioMed Online. 2013;27:201–11.
Franco JG, Baruffi RLR, Mauri AL, Petersen CG, Oliveira JBA, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod BioMed Online. 2008;17:42–5.
Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, et al. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58.
Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, et al. Correlation between DNA defect and sperm-head morphology. Reprod BioMed Online. 2012;24:211–8.
Franco Jr JG, Mauri AL, Petersen CG, Massaro FC, Silva LFI, Felipe V, et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl. 2011;35:46–51.
Cassuto NG, Montjean D, Siffroi J, Bouret D, Marzouk F, Copin H, et al. Different levels of DNA methylation detected in human sperms after morphological selection using high magnification microscopy. Biomed Res Int Hindawi Publishing Corporation. 2016;2016:1–7.
Wilding M, Coppola G, di Matteo L, Palagiano A, Fusco E, Dale B. Intracytoplasmic injection of morphologically selected spermatozoa (IMSI) improves outcome after assisted reproduction by deselecting physiologically poor quality spermatozoa. J Assist Reprod Genet. 2011;28:253–62.
Hammoud I, Boitrelle F, Ferfouri F, Vialard F, Bergere M, Wainer B, et al. Selection of normal spermatozoa with a vacuole-free head (x6300) improves selection of spermatozoa with intact DNA in patients with high sperm DNA fragmentation rates. Andrologia. 2013;45:163–70.
Utsuno H, Oka K, Yamamoto A, Shiozawa T. Evaluation of sperm head shape at high magnification revealed correlation of sperm DNA fragmentation with aberrant head ellipticity and angularity. Fertil Steril. 2013;99:1573–1580.e1.
Komiya A, Kato T, Kawauchi Y, Watanabe A, Fuse H. Clinical factors associated with sperm DNA fragmentation in male patients with infertility. ScientificWorldJournal. 2014;2014:868303.
Garolla A, Sartini B, Cosci I, Pizzol D, Ghezzi M, Bertoldo A, et al. Molecular karyotyping of single sperm with nuclear vacuoles identifies more chromosomal abnormalities in patients with testiculopathy than fertile controls: implications for ICSI. Hum Reprod. 2015;30:2493–500.
Vanderzwalmen P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod BioMed Online. 2008;17:617–27.
Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, et al. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25.
Knez K, Zorn B, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reprod Biol Endocrinol. 2011;9:123.
Knez K, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. Developmental dynamics of IMSI-derived embryos: a time-lapse prospective study. Reprod BioMed Online. 2013;27:161–71.
Neyer A, Zintz M, Stecher A, Bach M, Wirleitner B, Zech NH, et al. The impact of paternal factors on cleavage stage and blastocyst development analyzed by time-lapse imaging—a retrospective observational study. J Assist Reprod Genet. 2015;32:1607–14.
Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–9.
Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90.
Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod BioMed Online. 2006;12:634–8.
Kim HJ, Yoon HJ, Jang JM, Oh HS, Lee YJ, Lee WD, et al. Comparison between intracytoplasmic sperm injection and intracytoplasmic morphologically selected sperm injection in oligo-asthenoteratozoospermia patients. Clin Exp Reprod Med. 2014;41:9–14.
Shalom-Paz E, Anabusi S, Michaeli M, Karchovsky-Shoshan E, Rothfarb N, Shavit T, et al. Can intra cytoplasmatic morphologically selected sperm injection (IMSI) technique improve outcome in patients with repeated IVF-ICSI failure? A comparative study. Gynecol Endocrinol. 2015;31:247–51.
Hazout A, Dumont-Hassan M, Junca A-M, Bacrie PC, Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online. Reproductive Healthcare Ltd, Duck End Farm, Dry Drayton, Cambridge CB23 8DB, UK; 2006;12:19–25.
Antinori M, Licata E, Dani G, Cerusico F, Versaci C, D’Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod BioMed Online. 2008;16:835–41.
Klement AH, Koren-Morag N, Itsykson P, Berkovitz A. Intracytoplasmic morphologically selected sperm injection versus intracytoplasmic sperm injection: a step toward a clinical algorithm. Fertil Steril. 2013;99:1290–3.
Setti AS, Figueira RCS, Braga DPAF, Aoki T, Iaconelli A, Borges E. Intracytoplasmic morphologically selected sperm injection is beneficial in cases of advanced maternal age: a prospective randomized study. Eur J Obstet Gynecol Reprod Biol. 2013;171:286–90.
Knez K, Tomazevic T, Zorn B, Vrtacnik-Bokal E, Virant-Klun I. Intracytoplasmic morphologically selected sperm injection improves development and quality of preimplantation embryos in teratozoospermia patients. Reprod BioMed Online. 2012;25:168–79.
Cassuto NG, Hazout A, Bouret D, Balet R, Larue L, Benifla JL, et al. Low birth defects by deselecting abnormal spermatozoa before ICSI. Reprod BioMed Online. 2014;28:47–53.
Hershko-Klement A, Sukenik-Halevy R, Biron Shental T, Miller N, Berkovitz A. Intracytoplasmic morphologically selected sperm injection and congenital birth defects: a retrospective cohort study. Andrology. 2016;4:887–93.
Pinborg A, Henningsen A-KA, Malchau SS, Loft A. Congenital anomalies after assisted reproductive technology. Fertil Steril. 2013;99:327–32.
Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Sö derström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104.
Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333:679.
Tararbit K, Lelong N, Thieulin a-C, Houyel L, Bonnet D, Goffinet F, et al. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Hum Reprod. 2013;28:367–74.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Chaabane S, Sheehy O, Monnier P, Bissonnette F, Trasler JM, Fraser W, et al. Ovarian stimulators, intrauterine insemination, and assisted reproductive technologies use and the risk of major congenital malformations—the AtRISK study. Dev Reprod Toxicol. 2016;107:136–47.
Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk factors for birth defects. Benjamin Obstet Gynecol Surv. 2017;72:123–35.
Loane M, Dolk H, Morris JK. Maternal age-specific risk of non-chromosomal anomalies. BJOG An Int J Obstet Gynaecol. 2009;116:1111–9.
Nybo Andersen A-M, Urhoj SK. Is advanced paternal age a health risk for the offspring? Fertil Steril. 2017;107:312–8.
Sokal R, Tata LJ, Fleming KM. Sex prevalence of major congenital anomalies in the United Kingdom: a national population-based study and international comparison meta-analysis. Birth Defects Res A Clin Mol Teratol. 2014;100:79–91.
Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97:1331–1337.e4.
Silver R, Rodriguez R, Chang T, Gearhart J. In vitro fertilization is associated with an increased risk of hypospadias. J Urol. 1999;161:1954–7.
Fauser BCJM, Devroey P, Diedrich K, Balaban B, Bonduelle M, Delemarre-van de Waal HA, et al. Health outcomes of children born after IVF/ICSI: a review of current expert opinion and literature. Reprod BioMed Online. 2014;28:162–82.
Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42.
Barratt CLR, Aitken RJ, Björndahl L, Carrell DT, De Boer P, Kvist U, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications-a position report. Hum Reprod. 2010;25:824–38.
Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287–301.
Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–26.
Oliva R, Luís Ballescà J. Altered histone retention and epigenetic modifications in the sperm of infertile men. Asian J Androl. 2012;14:239–40.
Jenkins TG, Carrell DT. Dynamic alterations in the paternal epigenetic landscape following fertilization. Front Genet. 2012;3:1–8.
Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, Sokol RZ. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One. 2007;2:e1289.
Schagdarsurengin U, Paradowska A, Steger K. Analysing the sperm epigenome: roles in early embryogenesis and assisted reproduction. Nat Rev Urol. 2012;9:609–19.
Feinberg JI, Bakulski KM, Jaffe AE, Tryggvadottir R, Brown SC, Goldman LR, et al. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. Int J Epidemiol. 2015;44:1199–210.
Kobayashi H, Hiura H, John RM, Sato A, Otsu E, Kobayashi N, et al. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet. 2009;17:1582–91.
Acknowledgments
The authors thank Dr S Bulk from the Department of Genetics (Centre Hospitalier Universitaire de Liège) in Belgium for her help regarding the analysis of the babies’ neonatal data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gaspard, O., Vanderzwalmen, P., Wirleitner, B. et al. Impact of high magnification sperm selection on neonatal outcomes: a retrospective study. J Assist Reprod Genet 35, 1113–1121 (2018). https://doi.org/10.1007/s10815-018-1167-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1167-8