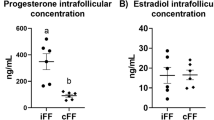
Abstract
Purpose
We hypothesized that the chemokine SDF1/CXCR4 system was present in feline cumulus-oocyte complexes (COCs) and that COCs cultured with SDF1 would directly upregulate gene expression in the ovulatory cascade.
Methods
Ovaries (n = 50) were obtained from adult domestic cats during the breeding season and COCs were recovered from antral follicles. Because IVM media triggers cumulus-oocyte expansion, culture conditions needed to be optimized to study periovulatory genes. After optimization, the effects of 25 ng/ml SDF1 and the CXCR4 inhibitor were examined in a COC culture for 3, 12, and 24 h.
Results
MEM-hepes with 1% of charcoal stripped-FBS was the optimized culture medium, assessed by the expansion of COCs at 24 h in the gonadotropin (GNT) group but not in the media with serum alone. The mRNA expression of HAS2, TNFAIP6, PTX3, and AREG peaked at 3 h in GNT group as compared to all other groups (p < 0.05). COCs cultured with SDF1 showed increased HAS2 and TNFAIP6 mRNA expression at 3 h compared to negative controls and to the CXCR4 inhibitor group. CXCR4 and SDF1 immunostaining was present in both cumulus cells and the oocyte.
Conclusions
These results demonstrate that GNT stimulation upregulates key periovulatory genes and expansion in feline COCs from antral follicles, and support the use of this culture system to examine molecular processes within the COC. In addition, SDF1 directly promotes key periovulatory genes in feline COCs, suggesting that the SDF1-CXCR4 pathway may extend its function beyond a chemoattractant, and may play a direct role within the COC.




Similar content being viewed by others
References
Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N. Lessons from biodiversity—the value of nontraditional species to advance reproductive science, conservation, and human health. Mol Reprod Dev. 2010;77:397–409. https://doi.org/10.1002/mrd.21137.
Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–54. https://doi.org/10.1002/mrd.20769.
Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–16.
Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93. https://doi.org/10.1002/mrd.1080340114.
Hess KA, Chen L, Larsen WJ. Inter-alpha-inhibitor binding to hyaluronan in the cumulus extracellular matrix is required for optimal ovulation and development of mouse oocytes. Biol Reprod. 1999;61:436–43.
Salustri A, Garlanda C, Hirsch E, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–86. https://doi.org/10.1242/dev.01056.
Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–9. https://doi.org/10.1016/j.mce.2005.01.004.
Chen L, Mao SJ, Larsen WJ. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-alpha-trypsin inhibitor family. J Biol Chem. 1992;267:12380–6.
Fulop C, Szanto S, Mukhopadhyay D, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–61.
Mukhopadhyay D, Hascall VC, Day AJ, et al. Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch Biochem Biophys. 2001;394:173–81. https://doi.org/10.1006/abbi.2001.2552.
Peng J, Li Q, Wigglesworth K, et al (2013) Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A 110:E776-85. doi: https://doi.org/1218020110 [pii] https://doi.org/10.1073/pnas.1218020110.
Eppig JJ. Prostaglandin E2 stimulates cumulus expansion and hyaluronic acid synthesis by cumuli oophori isolated from mice. Biol Reprod. 1981;25:191–5.
Tamba S, Yodoi R, Segi-Nishida E, et al. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc Natl Acad Sci U S A. 2008;105:14539–44. https://doi.org/10.1073/pnas.0805699105.
Yodoi R, Tamba S, Morimoto K, et al. RhoA/Rho kinase signaling in the cumulus mediates extracellular matrix assembly. Endocrinology. 2009;150:3345–52. https://doi.org/10.1210/en.2008-1449.
Skinner MK, Schmidt M, Savenkova MI, et al. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–72. https://doi.org/10.1002/mrd.20883.
Sayasith K, Sirois J. Expression and regulation of stromal cell-derived factor-1 (SDF1) and chemokine CXC motif receptor 4 (CXCR4) in equine and bovine preovulatory follicles. Mol Cell Endocrinol. 2014;391:10–21. https://doi.org/10.1016/j.mce.2014.04.009.
Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, et al. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–21. https://doi.org/10.1210/me.2005-0420.
Rojo JL, Linari M, Musse MP, Peluffo MC. Felis catus ovary as a model to study follicle biology in vitro. J Assist Reprod Genet. 2015;32:1105–11. https://doi.org/10.1007/s10815-015-0511-5.
Peluffo MC, Stanley J, Braeuer N, et al. A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Hum Reprod. 2014;29:1400–12. https://doi.org/10.1093/humrep/deu083.
Peluffo MC, Barrett SL, Stouffer RL, et al. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod. 2010;83:525–32. https://doi.org/10.1095/biolreprod.110.084418.
Peluffo MC, Ting AY, Zamah AM, et al (2012) Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. doi: https://doi.org/10.1093/humrep/des158.
Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–16.
Gomez MC, Pope E, Harris R, et al. Development of in vitro matured, in vitro fertilized domestic cat embryos following cryopreservation, culture and transfer. Theriogenology. 2003;60:239–51.
Xu F, Stouffer RL, Muller J, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–65. https://doi.org/10.1093/molehr/gaq089.
Park JY, Su YQ, Ariga M, et al (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science (80- ) 303:682–684. doi: doi:https://doi.org/10.1126/science.1092463.
Jamnongjit M, Gill A, Hammes SR (2005) Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci U S A 102:16257–16262. doi: https://doi.org/10.1073/pnas.0508521102.
Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–65. https://doi.org/10.1210/me.2005-0504.
Bristol SK, Woodruff TK. Follicle-restricted compartmentalization of transforming growth factor beta superfamily ligands in the feline ovary. Biol Reprod. 2004;70:846–59. https://doi.org/10.1095/biolreprod.103.021857.
Ochsner SA, Russell DL, Day AJ, et al. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003;144:1008–19.
Holt JE, Jackson A, Roman SD, et al. CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev Biol. 2006;293:449–60. https://doi.org/10.1016/j.ydbio.2006.02.012.
McKenzie LJ, Pangas SA, Carson SA, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–74. https://doi.org/10.1093/humrep/deh535.
Goodrowe KL, Wall RJ, O’Brien SJ, et al. Developmental competence of domestic cat follicular oocytes after fertilization in vitro. Biol Reprod. 1988;39:355–72.
Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum Reprod. 2011;26:2165–77. https://doi.org/10.1093/humrep/der176.
Acknowledgements
We are grateful to Olga Bustamante from the “Centro de Sanidad Animal de la Municipalidad de Merlo” (Provincia de Buenos Aires) for the donation of the feline ovaries. Recombinant human FSH and LH (Merck Serono) were generously donated for this project. A special thanks to German La Iacona for his technical assistance with the confocal microscope.
Funding
This study was supported PRESTAMO BID PICT 2014 N° 666 (MCP), Small Faculty Grants Program CNSM CSULB (KAY), the CSULB Professors Around the World Award (KAY), and by the Fogarty International Center, of the National Institutes of Health under Award R01TW009163 (MCP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Online Resource 1
COCs cultured for 12 h with 25 ng/ml SDF1 showed no changes (P > 0.05) in (a) HAS2, (b) TNFAIP6, (c) PTX3, (d) AREG and (e) GDF9 expression at 12 h between SDF1 and control treated groups. Bars represent the real-time PCR relative-quantitation of mRNA levels normalized to 18S (mean ± SEM; n = 4/time point). P values for the analysis of each gene are listed in each corresponding graph. (PDF 38 kb)
Online Resource 2
COCs cultured for 24 h with 25 ng/ml SDF1 showed no changes (P > 0.05) in (a) HAS2, (b) TNFAIP6, (c) PTX3, (d) AREG and (e) GDF9 expression at 24 h between SDF1 and control treated groups. Bars represent the real-time PCR relative-quantitation of mRNA levels normalized to 18S (mean ± SEM; n = 4/time point). P values for the analysis of each gene are listed in each corresponding graph. (PDF 40 kb)
Rights and permissions
About this article
Cite this article
Rojo, J.L., Linari, M., Young, K.A. et al. Stromal-derived factor 1 directly promotes genes expressed within the ovulatory cascade in feline cumulus oocyte complexes. J Assist Reprod Genet 35, 785–792 (2018). https://doi.org/10.1007/s10815-018-1150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1150-4