Abstract
Purpose
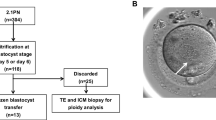
In this study, we examined the correlation between pronucleus size and the potential for human single pronucleus (1PN) zygotes to develop into blastocysts after IVF and ICSI.
Methods
This study included 112 patients who underwent a total of 112 cycles of IVF/ICSI. To evaluate embryo development, 1PN zygotes were compared with 2PN zygotes in the same IVF/ICSI cycle (control cycles) using time-lapse live embryo imaging. To assess the potential for blastocyst formation, cutoff values for pronuclear area and diameter were established through receiver operating characteristic curve analysis, after which 1PN zygotes were classified based on those cutoff values.
Results
Among 1PN zygotes cultured to day 5/6, the rate of embryo development was significantly lower than from 2PN zygotes. However, the rates of blastocyst formation and good quality blastocysts from 1PN zygotes with large pronuclear areas (≥ 710 μm2) or diameters (≥ 31 μm) were significantly higher than from 1PN zygotes with smaller pronuclear areas (≤ 509, 510–609, and 610–709 μm2) or diameters (≤ 24, 25–27,and 28–30 μm) (P < 0.01). Moreover, the results for 1PN zygotes with large pronuclei were similar to those for 2PN zygotes.
Conclusions
The developmental potential of 1PN zygotes with large pronuclear areas (≥ 710 μm2) or diameters (31 μm) appears to be similar to that of 2PN zygotes, and measurement of pronuclear area or diameter in 1PN zygotes is a simple, potentially useful, clinical method.


Similar content being viewed by others
References
Staessen C, Janssenswillen C, Devroey P, Van Steirteghem AC. Cytogenetic and morphological observations of single pronucleated human oocytes after in-vitro fertilization. Hum Reprod. 1993;8:221–3.
Reichman DE, Jackson KV, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94:965–70.
Gras L, Trounson AO. Pregnancy and birth resulting from transfer of a blastocyst observed to have one pronucleus at the time of examination for fertilization. Hum Reprod. 1999;14:1869–71.
Dasig D, Lyon J, Behr B, Milki AA. Monozygotic twin birth after the transfer of a cleavage stage embryo resulting from a single pronucleated oocyte. J Assist Reprod Genet. 2004;21:427–9.
Itoi F, Asano Y, Shimizu M, Honnma H, Murata Y. Birth of nine normal healthy babies following transfer of blastocysts derived from human single-pronucleate zygotes. J Assist Reprod Genet. 2015;32:1401–7.
Bradley CK, Traversa MV, Hobson N, Gee AJ, McArthur SJ. Clinical use of monopronucleated zygotes following blastocyst culture and preimplantation genetic screening, including verification of biparental chromosome inheritance. Reprod BioMed Online. 2017;34:567–74.
Mateo S, Vidal F, Parriego M, Rodríguez I, Montalvo V, Veiga A, et al. Could monopronucleated ICSI zygotes be considered for transfer? Analysis through time-lapse monitoring and PGS. J Assist Reprod Genet. 2017;34:905–11.
Staessen C, Van Steirteghem AC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12:321–7.
Sultan KM, Munne S, Palermo GD, Alikani M, Cohen J. Chromosomal status of uni-pronuclear human zygotes following in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1995;10:132–6.
Yan J, Li Y, Shi Y, Feng HL, Gao S, Chen ZJ. Assessment of sex chromosomes of human embryos arising from monopronucleus zygotes in in vitro fertilization and intracytoplasmic sperm injection cycles of Chinese women. Gynecol Obstet Investig. 2010;69:20–3.
van der Heijden GW, van den Berg IM, Baart EB, Derijck AA, Martini E, de Boer P. Parental origin of chromatin in human monopronuclear zygotes revealed by asymmetric histone methylation patterns, differs between IVF and ICSI. Mol Reprod Dev. 2009;76:101–8.
Otsu E, Sato A, Nagaki M, Araki Y, Utsunomiya T. Developmental potential and chromosomal constitution of embryos derived from larger single pronuclei of human zygotes used in in vitro fertilization. Fertil Steril. 2004;81:723–4.
Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;5:617–31.
Itoi F, Asano Y, Shimizu M, Nagai R, Saitou K, Honnma H, et al. Clinical outcomes after IVF or ICSI using human blastocysts derived from oocytes containing aggregates of smooth endoplasmic reticulum. Reprod BioMed Online. 2017;34:337–44.
Veeck LL. An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999.
Schoolcraft WB, Gardner DK, Lane M, Schlenker T, Hamilton F, Meldrum DR. Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril. 1999;72:604–9.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Capalbo A, Treff N, Cimadomo D, Tao X, Ferrero S, Vaiarelli A, et al. Abnormally fertilized oocytes can result in healthy live births: improved genetic technologies for preimplantation genetic testing can be used to rescue viable embryos in in vitro fertilization cycles. Fertil Steril. 2017;108:1007–15.
Mateo S, Parriego M, Boada M, Vidal F, Coroleu B, Veiga A. In vitro development and chromosome constitution of embryos derived from monopronucleated zygotes after intracytoplasmic sperm injection. Fertil Steril. 2013;99:897–902.
Levron J, Munne S, Willadsen S, Rosenwaks Z, Cohen J. Male and female genomes associated in a single pronucleus in human zygotes. Biol Reprod. 1995;52:653–7.
Krukowska A, Tarkowski AK. Mouse zygotes with one diploid pronucleus formed as a result of ICSI can develop normally beyond birth. Mol Reprod Dev. 2005;72:346–53.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12:532–41.
Acknowledgements
We thank the embryology staff of the Kishokai Medical Corporation for their assistance in preparing this manuscript and for their expert technical help.
Funding
This work was no external funding.
Author information
Authors and Affiliations
Contributions
EA acquired the data, performed the statistical analysis, and helped to draft the manuscript. FI conceived and designed the study, acquired the data, performed the statistical analysis, and drafted the manuscript. YA acquired the data and helped to draft the manuscript. HH performed the statistical analysis and drafted the manuscript. HO drafted the manuscript. KN participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Kishokai Medical Corporation and was conducted in accordance with the principals expressed in the Declaration of Helsinki. All patients provided consent to all treatment procedures and agreed to anonymous use of their data for studies.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Araki, E., Itoi, F., Honnma, H. et al. Correlation between the pronucleus size and the potential for human single pronucleus zygotes to develop into blastocysts. J Assist Reprod Genet 35, 817–823 (2018). https://doi.org/10.1007/s10815-018-1137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1137-1