Abstract
Purpose
Allogeneic ovarian transplantation may be an alternative in the future to oocyte donation in women with premature ovarian failure. The objectives of this study were to (a) evaluate allotransplantation feasibility for restoration of ovarian function and (b) assess efficacy of synthetic preimplantation factor (PIF) monotherapy as sole immune-acceptance regimen.
Methods
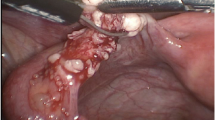
This is an experimental animal study using non-human primates (Papio anubis). Allogeneic orthotopic ovarian tissue transplantation was performed in two female olive baboons. PIF was administered as a monotherapy to prevent immune rejection and achieve transplant maintenance and function. Subjects underwent bilateral oophorectomy followed by cross-transplantation of prepared ovarian cortex. Postoperatively, subjects were monitored for clinical and biochemical signs of graft rejection and return of function. Weekly blood samples were obtained to monitor graft acceptance and endocrine function restoration.
Results
Postoperatively, there were no clinical signs of rejection. Laboratory parameters (alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine) did not indicate organ rejection at any stage of the experiment. Initially, significant loss of follicles was noticed after grafting and serum follicle-stimulating hormone (FSH) and E2 levels were consistent with ovarian failure. Seven months after transplantation, one animal exhibited recurrence of ovarian endocrine function (perineal swelling, E2 rise, FSH decrease, and return of menstruation).
Conclusions
Organ rejection after allogeneic ovarian transplantation was prevented using PIF as monotherapy for the first time and no side effects were recorded. The study suggests the clinical feasibility of ovarian allotransplantation to obtain ovarian function.







Similar content being viewed by others
References
Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85(1):1–11. https://doi.org/10.1016/j.fertnstert.2005.04.071.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. https://doi.org/10.1016/S0140-6736(04)17222-X.
Scott JR, Hendrickson M, Lash S, Shelby J. Pregnancy after tubo-ovarian transplantation. Obstet Gynecol. 1987;70(2):229–34.
Carmona F, Balasch J, Gonzalez-Merlo J. Ovarian function, tubal viability and pregnancy after tubo-ovarian transplantation in the rabbit. Hum Reprod. 1993;8(6):929–31.
Kim SS, Battaglia DE, Soules MR. The future of human ovarian cryopreservation and transplantation: fertility and beyond. Fertil Steril. 2001;75(6):1049–56.
Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet. 1999;353(9158):1083–91. https://doi.org/10.1016/S0140-6736(98)07493-5.
Mhatre P, Mhatre J. Orthotopic ovarian transplant—review and three surgical techniques. Pediatr Transplant. 2006;10(7):782–7. https://doi.org/10.1111/j.1399-3046.2006.00547.x.
Mhatre P, Mhatre J, Magotra R. Ovarian transplant: a new frontier. Transplant Proc. 2005;37(2):1396–8. https://doi.org/10.1016/j.transproceed.2004.11.083.
Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–6. https://doi.org/10.1038/ni1317.
Barnea ER. Insight into early pregnancy events: the emerging role of the embryo. Am J Reprod Immunol. 2004;51(5):319–22. https://doi.org/10.1111/j.1600-0897.2004.00159.x.
Barnea ER. Applying embryo-derived immune tolerance to the treatment of immune disorders. Ann N Y Acad Sci. 2007;1110:602–18. https://doi.org/10.1196/annals.1423.064.
Barnea ER. Signaling between embryo and mother in early pregnancy: basis for development of tolerance. In: Carp HJA, editor. Recurrent pregnancy loss—causes, controversies, and treatment. Second ed. Boca Raton: CRC Press; 2014. p. 17–28.
Barnea ER, Rambaldi M, Paidas MJ, Mecacci F. Reproduction and autoimmune disease: important translational implications from embryo-maternal interaction. Immunotherapy. 2013;5(7):769–80. https://doi.org/10.2217/imt.13.59.
Stamatkin CW, Roussev RG, Stout M, Absalon-Medina V, Ramu S, Goodman C, et al. Preimplantation factor (PIF) correlates with early mammalian embryo development-bovine and murine models. Reproductive biology and endocrinology : RB&E. 2011;9:63. https://doi.org/10.1186/1477-7827-9-63.
Stamatkin CW, Roussev RG, Stout M, Coulam CB, Triche E, Godke RA, et al. Preimplantation factor negates embryo toxicity and promotes embryo development in culture. Reprod BioMed Online. 2011;23(4):517–24. https://doi.org/10.1016/j.rbmo.2011.06.009.
Barnea ER, Hayrabedyan S, Todorova K, Almogi-Hazan O, Or R, Guingab J, et al. Preimplantation factor (PIF*) regulates systemic immunity and targets protective regulatory and cytoskeleton proteins. Immunobiology. 2016;221(7):778–93. https://doi.org/10.1016/j.imbio.2016.02.004.
Chen YC, Rivera J, Fitzgerald M, Hausding C, Ying YL, Wang X, et al. Preimplantation factor prevents atherosclerosis via its immunomodulatory effects without affecting serum lipids. Thromb Haemost. 2016;115(5):1010–24. https://doi.org/10.1160/TH15-08-0640.
Weiss L, Bernstein S, Jones R, Amunugama R, Krizman D, Jebailey L, et al. Preimplantation factor (PIF) analog prevents type I diabetes mellitus (TIDM) development by preserving pancreatic function in NOD mice. Endocrine. 2011;40(1):41–54. https://doi.org/10.1007/s12020-011-9438-5.
Weiss L, Or R, Jones RC, Amunugama R, JeBailey L, Ramu S, et al. Preimplantation factor (PIF*) reverses neuroinflammation while promoting neural repair in EAE model. J Neurol Sci. 2012;312(1–2):146–57. https://doi.org/10.1016/j.jns.2011.07.050.
Mueller M, Schoeberlein A, Zhou J, Joerger-Messerli M, Oppliger B, Reinhart U, et al. Preimplantation factor bolsters neuroprotection via modulating protein kinase A and protein kinase C signaling. Cell Death Differ. 2015;22(12):2078–86. https://doi.org/10.1038/cdd.2015.55.
Mueller M, Zhou J, Yang L, Gao Y, Wu F, Schoeberlein A, et al. Preimplantation factor promotes neuroprotection by targeting microRNA let-7. Proc Natl Acad Sci U S A. 2014;111(38):13882–7. https://doi.org/10.1073/pnas.1411674111.
Shainer R, Almogi-Hazan O, Berger A, Hinden L, Mueller M, Brodie C, et al. Preimplantation factor (PIF) therapy provides comprehensive protection against radiation induced pathologies. Oncotarget. 2016;7(37):58975–94. 10.18632/oncotarget.10635.
Azar Y, Shainer R, Almogi-Hazan O, Bringer R, Compton SR, Paidas MJ, et al. Preimplantation factor reduces graft-versus-host disease by regulating immune response and lowering oxidative stress (murine model). Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19(4):519–28. https://doi.org/10.1016/j.bbmt.2012.12.011.
Shainer R, Azar Y, Almogi-Hazan O, Bringer R, Compton SR, Paidas MJ, et al. Immune regulation and oxidative stress reduction by preimplantation factor following syngeneic or allogeneic bone marrow transplantation. Conference Papers in Medicine. 2013;2013(Article ID 718031):1–8. https://doi.org/10.1155/2013/718031.
Barnea ER, Almogi-Hazan O, Or R, Mueller M, Ria F, Weiss L, et al. Immune regulatory and neuroprotective properties of preimplantation factor: from newborn to adult. Pharmacol Ther. 2015;156:10–25. https://doi.org/10.1016/j.pharmthera.2015.10.008.
Johannesson L, Enskog A, Molne J, Diaz-Garcia C, Hanafy A, Dahm-Kahler P, et al. Preclinical report on allogeneic uterus transplantation in non-human primates. Hum Reprod. 2013;28(1):189–98. https://doi.org/10.1093/humrep/des381.
Enskog A, Johannesson L, Chai DC, Dahm-Kahler P, Marcickiewicz J, Nyachieo A, et al. Uterus transplantation in the baboon: methodology and long-term function after auto-transplantation. Hum Reprod. 2010;25(8):1980–7. https://doi.org/10.1093/humrep/deq109.
Di Simone N, Di Nicuolo F, Marana R, Castellani R, Ria F, Veglia M, et al. Synthetic preimplantation factor (PIF) prevents fetal loss by modulating LPS induced inflammatory response. PLoS One. 2017;12(7):e0180642. https://doi.org/10.1371/journal.pone.0180642.
Migliara G, Mueller M, Piermattei A, Brodie C, Paidas MJ, Barnea ER, et al. PIF* promotes brain re-myelination locally while regulating systemic inflammation- clinically relevant multiple sclerosis M.smegmatis model. Oncotarget. 2017;8(13):21834–51. 10.18632/oncotarget.15662.
Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77(4):303–7. https://doi.org/10.1016/j.contraception.2008.01.003.
Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153(7):3100–10. https://doi.org/10.1210/en.2011-2111.
Bauer C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception. 2015;92(2):120–3. https://doi.org/10.1016/j.contraception.2015.06.007.
Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–70. https://doi.org/10.1007/s10815-015-0544-9.
Brannstrom M, Johannesson L, Bokstrom H, Kvarnstrom N, Molne J, Dahm-Kahler P, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–16. https://doi.org/10.1016/S0140-6736(14)61728-1.
Johannesson L, Kvarnstrom N, Molne J, Dahm-Kahler P, Enskog A, Diaz-Garcia C, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199–204. https://doi.org/10.1016/j.fertnstert.2014.09.024.
Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003;18(6):1165–72.
Gosden RG. Survival of ovarian allografts in an experimental animal model. Pediatr Transplant. 2007;11(6):628–33. https://doi.org/10.1111/j.1399-3046.2007.00715.x.
Meraz MM, Juarez CG, Monsalve CR, Martinez-Chequer JC, Duvignau JM, Fernandez EM, et al. Restoration of endocrine function and fertility with orthotopic tubal-ovarian allotransplant as the anatomical-functional unit in rabbits. Journal of investigative surgery : the official journal of the Academy of Surgical Research. 2008;21(6):348–59. https://doi.org/10.1080/08941930802438930.
Lin YH, Yeh YC, Tzeng CR, Shang WJ, Liu JY, Chen CH. Evaluating the effects of immunosuppression by in-vivo bioluminescence imaging after allotransplantation of ovarian grafts. Reprod BioMed Online. 2011;22(2):220–7. https://doi.org/10.1016/j.rbmo.2010.10.010.
Chen CH, Yeh YC, Wu GJ, Huang YH, Lai WF, Liu JY, et al. Tracking the rejection and survival of mouse ovarian iso- and allografts in vivo with bioluminescent imaging. Reproduction. 2010;140(1):105–12. https://doi.org/10.1530/REP-09-0448.
Maclaran K, Panay N. Premature ovarian failure. The journal of family planning and reproductive health care. 2011;37(1):35–42. https://doi.org/10.1136/jfprhc.2010.0015.
van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–8.
Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. The Lancet Oncology. 2006;7(10):821–8. https://doi.org/10.1016/S1470-2045(06)70869-5.
Popat VB, Calis KA, Vanderhoof VH, Cizza G, Reynolds JC, Sebring N, et al. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab. 2009;94(7):2277–83. https://doi.org/10.1210/jc.2008-1878.
Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, et al. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J Clin Endocrinol Metab. 2011;96(2):E278–87. https://doi.org/10.1210/jc.2010-0613.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–92.
Asch R, Balmaceda J, Ord T, Borrero C, Cefalu E, Gastaldi C, et al. Oocyte donation and gamete intrafallopian transfer as treatment for premature ovarian failure. Lancet. 1987;1(8534):687.
Vujovic S, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, Moen MH, et al. EMAS position statement: managing women with premature ovarian failure. Maturitas. 2010;67(1):91–3. https://doi.org/10.1016/j.maturitas.2010.04.011.
Moindjie H, Santos ED, Loeuillet L, Gronier H, de Mazancourt P, Barnea ER, et al. Preimplantation factor (PIF) promotes human trophoblast invasion. Biol Reprod. 2014;91(5):118. https://doi.org/10.1095/biolreprod.114.119156.
Barnea ER, Simon J, Levine SP, Coulam CB, Taliadouros GS, Leavis PC. Progress in characterization of pre-implantation factor in embryo cultures and in vivo. Am J Reprod Immunol. 1999;42(2):95–9.
Roussev RG, Dons'koi BV, Stamatkin C, Ramu S, Chernyshov VP, Coulam CB, et al. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reprod BioMed Online. 2013;26(1):79–87. https://doi.org/10.1016/j.rbmo.2012.09.017.
Lopez-Cabrera M, Santis AG, Fernandez-Ruiz E, Blacher R, Esch F, Sanchez-Mateos P, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178(2):537–47.
Schowengerdt KO, Fricker FJ, Bahjat KS, Kuntz ST. Increased expression of the lymphocyte early activation marker CD69 in peripheral blood correlates with histologic evidence of cardiac allograft rejection. Transplantation. 2000;69(10):2102–7.
Posselt AM, Vincenti F, Bedolli M, Lantz M, Roberts JP, Hirose R. CD69 expression on peripheral CD8 T cells correlates with acute rejection in renal transplant recipients. Transplantation. 2003;76(1):190–5. https://doi.org/10.1097/01.TP.0000073614.29680.A8.
Stevens VC. Some reproductive studies in the baboon. Hum Reprod Update. 1997;3(6):533–40.
D’Hooghe T, Mwenda JM, Hill JA. The baboon as a nonhuman primate model for the study of human reproduction. Gynecol Obstet Invest. 2004;57:1–60.
Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28(8):2146–56. https://doi.org/10.1093/humrep/det103.
Nyachieo A, Spiessens C, Chai DC, Kiulia NM, Willemen D, Mwenda JM, et al. Ovarian tissue cryopreservation by vitrification in olive baboons (Papio anubis): a pilot study. Gynecol Obstet Investig. 2013;75(3):157–62. https://doi.org/10.1159/000346084.
Todo S, Demetris A, Ueda Y, Imventarza O, Cadoff E, Zeevi A, et al. Renal transplantation in baboons under FK 506. Surgery. 1989;106(2):444–50. discussion 50-1
Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82(3):679–85. https://doi.org/10.1016/j.fertnstert.2004.05.022.
Robertson JA. Ethical issues in ovarian transplantation and donation. Fertil Steril. 2000;73(3):443–6.
Acknowledgements
The present study was presented at the late-breaking abstract session of the ASRM annual meeting in Salt-Lake City, October 19th 2016. The authors wish to thank Ms. Sharon Chepkwony and Mr. Nicholas Kiulia for their organizational help at the Institute of Primate Research (IPR), Karen, Kenya, and Dr. David Erikson for hormonal assays at the Oregon National Primate Research Center, OR, Portland. In addition, we appreciate Amy Carter and Stephanie Zinn for editorial assistance.
Funding
This study was funded by the Medical Scientific Fund of the Mayor of Vienna (Nr.:14063), the Wunschbaby Institut Feichtinger, by an unrestricted grant from BioIncept and by private funds of M.F. and S.S.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute of Primate Research (IPR), Karen, Kenya (Ref.: IRC/10/14).
Rights and permissions
About this article
Cite this article
Feichtinger, M., Barnea, E.R., Nyachieo, A. et al. Allogeneic ovarian transplantation using immunomodulator preimplantation factor (PIF) as monotherapy restored ovarian function in olive baboon. J Assist Reprod Genet 35, 81–89 (2018). https://doi.org/10.1007/s10815-017-1051-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1051-y