Abstract
Purpose
Polycystic ovary syndrome (PCOS) is the most common cause of ovulatory dysfunction and female infertility. The etiopathogenetic mechanisms of PCOS have been studied for many years, although exact causes remain unclear. It has been demonstrated that proteoglycan degradation by a disintegrin-like metalloproteinase with thrombospondin type motifs-1 (ADAMTS-1) is essential for ovulation and fertilization. The objective of our study is to analyze the levels of ADAMTS-1 and aggrecan in the follicular fluid (FF) of PCOS patients compared with normal ovulatory women and to determine whether these markers could be a predictor of in vitro fertilization (IVF) success in PCOS patients.
Methods
Women with PCOS (n = 21) and normal ovulatory controls (n = 22) undergoing IVF treatment were recruited. ADAMTS-1 and aggrecan levels were analyzed with enzyme-linked immunosorbent assay (ELISA) and compared between PCOS and normal ovulatory controls. The predictor effect of ADAMTS-1 and aggrecan on fertilization rate and implantation was evaluated.
Results
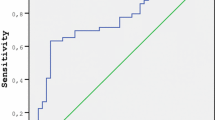
FF ADAMTS-1 and aggrecan levels increased in women with PCOS compared to controls. Elevated ADAMTS-1 levels but not aggrecan were related to increased implantation in PCOS.
Conclusion
Our study demonstrated that altered levels of ADAMTS-1 and aggrecan may have a partial role in the etiopathogenesis of PCOS, and ADAMTS-1 could be a predictive marker for implantation success in PCOS patients.

Similar content being viewed by others
References
Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:304–11.
Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–9.
Corbett S, Morin-Papunen L. The polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol. 2013;373:39–50.
Yasuo T, Yamaguchi T, Kitaya K. Progesterone induction of chondroitin sulfate proteoglycan aggrecan expression in human endometrial epithelial cells. J Steroid Biochem Mol Biol. 2010;122:159–63.
Yanagishita M, Hascall VC. Biosynthesis of proteoglycans by rat granulosa cells cultured in vitro. J Biol Chem. 1979;254:12355–64.
Mueller PL, Schreiber JR, Lucky AW, Schulman JD, Rodbard D, Ross GT. Follicle-stimulating hormone stimulates ovarian synthesis of proteoglycans in the estrogen-stimulated hypophysectomized immature female rat. Endocrinology. 1978;102:824–31.
Yanagishita M. Proteoglycans and hyaluronan in female reproductive organs. EXS. 1994;70:179–90.
Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta. 1812;2011:1616–29.
Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312.
Brown HM, Dunning KM, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83:549–57.
Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–8.
Xiao S, Li Y, Chen M, Xu Y, Wen Y, Zhou C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J Clin Endocrinol Metab. 2014;99:1015–21.
Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709.
Doyle KM, Russell DL, Sriraman V, Richards JS. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol. 2004;18:2463–78.
Boerboom D, Russell DL, Richards JS, Sirois J. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin-like motifs-1) and progesterone receptor by human chorionic gonadotropin in equine preovulatory follicles. J Mol Endocrinol. 2003;31:473–85.
Russell, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144:1020–31.
Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–27.
Yung Y, Maman E, Konopnicki S, Cohen B, Brengauz M, Lojkin I, et al. ADAMTS-1: a new human ovulatory gene and a cumulus marker for fertilization capacity. Mol Cell Endocrinol. 2010;328:104–8.
Eriksen GV, Malmstrom A, Uldbjerg N. Human follicular fluid proteoglycans in relation to in vitro fertilization. Fertil Steril. 1997;68(5):791–8.
Bellin ME, Ax RL, Laufer N, Tarlatzis BC, DeCherney AH, Feldberg D, et al. Glycosaminoglycans in follicular fluid from women undergoing in vitro fertilization and their relationship to cumulus expansion, fertilization, and development. Fertil Steril. 1986;45:244–8.
Acknowledgements
E.N.T. formulated the present hypothesis and was responsible for writing the report. D.U.K. and B.O. collected the data and M.E. was responsible for analyzing the data. N.K. was responsible for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tola, E.N., Karatopuk, D.U., Koroglu, N. et al. Follicular ADAMTS-1 and aggrecan levels in polycystic ovary syndrome. J Assist Reprod Genet 34, 811–816 (2017). https://doi.org/10.1007/s10815-017-0913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0913-7