Abstract
Purpose
The purpose of this study was to summarize the latest advances and successes in the field of ovarian tissue cryopreservation while identifying gaps in current knowledge that suggest opportunities for future research.
Methods
A systematic review was performed according to PRISMA guidelines for all relevant full-text articles in PubMed published in English that reviewed or studied historical or current advancements in ovarian tissue cryopreservation and auto-transplantation techniques.
Results
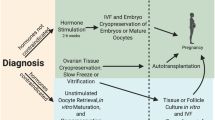
Ovarian tissue auto-transplantation in post-pubertal women is capable of restoring fertility with over 80 live births currently reported with a corresponding pregnancy rate of 23 to 37%. The recently reported successes of live births from transplants, both in orthotopic and heterotopic locations, as well as the emerging methods of in vitro maturation (IVM), in vitro culture of primordial follicles, and possibility of in vitro activation (IVA) suggest new fertility options for many women and girls. Vitrification, as an ovarian tissue cryopreservation technique, has also demonstrated successful live births and may be a more cost-effective method to freezing with less tissue injury. Further, transplantation via the artificial ovary with an extracellular tissue matrix (ECTM) scaffolding as well as the effects of sphingosine-1-phosphate (SIP) and fibrin modified with heparin-binding peptide (HBP), heparin, and a vascular endothelial growth factor (VEGF) have demonstrated important advancements in fertility preservation. As a fertility preservation method, ovarian tissue cryopreservation and auto-transplantation are currently considered experimental, but future research may pave the way for these modalities to become a standard of care for women facing the prospect of sterility from ovarian damage.

Similar content being viewed by others
References
International Agency for Research on Cancer. https://www.iarc.fr. 2016.
American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics.html. 2016.
Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–18. doi:10.1016/S1470-2045(05)70092-9.
Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121(10):1532–9. doi:10.1002/cncr.29181.
Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29(9):1931–40. doi:10.1093/humrep/deu158.
Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P. Updates in preserving reproductive potential of prepubertal girls with cancer: systematic review. Crit Rev Oncol Hematol. 2016;103:10–21. doi:10.1016/j.critrevonc.2016.04.002.
Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–9. doi:10.1093/humrep/dev128.
Dunlop CE, Anderson RA. Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–50. doi:10.1016/j.maturitas.2014.12.005.
Gunasheela S, Gunasheela D. Preventative management of infertility caused by treatment of malignancy. In: Gunasheela S, editor. Practical management of gynecological problems. 2nd ed. New Delhi: Jaypee Brothers Medical Pub; 2011. pp. 233–49.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39. doi:10.1097/GRF.0b013e3181f96b54.
Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr. 2005;34:64–8. doi:10.1093/jncimonographs/lgi022.
McCartney C, Marshall J. Neuroendocrinology of reproduction. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 3–26.
Hall J. Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 141–56.
Ogilvy-Stuart AL, Shalet SM. Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101(Suppl 2):109–16.
Critchley HO, Bath LE, Wallace WH. Radiation damage to the uterus—review of the effects of treatment of childhood cancer. Hum Fertil (Camb). 2002;5(2):61–6.
Waimey KE, Duncan FE, Su HI, Smith K, Wallach H, Jona K, et al. Future directions in oncofertility and fertility preservation: a report from the 2011 oncofertility consortium conference. J Adolesc Young Adult Oncol. 2013;2(1):25–30. doi:10.1089/jayao.2012.0035.
Practice Committee of American Society for Reproductive M. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–43. doi:10.1016/j.fertnstert.2014.02.052.
Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34(4):807–22. doi:10.1007/s10555-015-9600-2.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi:10.1016/j.fertnstert.2013.03.030.
Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):87–100. doi:10.1016/j.bpobgyn.2009.09.003.
Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology. 1999;38(4):301–9. doi:10.1006/cryo.1999.2170.
Hunter J, Bernard A, Fuller B, McGrath J, Shaw RW. Plasma membrane water permeabilities of human oocytes: the temperature dependence of water movement in individual cells. J Cell Physiol. 1992;150(1):175–9. doi:10.1002/jcp.1041500123.
Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54(1):102–8.
Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing—a review article. Mol Cell Endocrinol. 2003;202(1–2):101–7.
Glenister PH, Wood MJ, Kirby C, Whittingham DG. Incidence of chromosome anomalies in first-cleavage mouse embryos obtained from frozen-thawed oocytes fertilized in vitro. Gamete Res. 1987;16(3):205–16. doi:10.1002/mrd.1120160303.
Johnson MH, Pickering SJ, George MA. The influence of cooling on the properties of the zona pellucida of the mouse oocyte. Hum Reprod. 1988;3(3):383–7.
Gook DA, Osborn SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Hum Reprod. 1994;9(4):684–91.
Ragni G, Allegra A, Anserini P, Causio F, Ferraretti AP, Greco E, et al. The 2004 Italian legislation regulating assisted reproduction technology: a multicentre survey on the results of IVF cycles. Hum Reprod. 2005;20(8):2224–8. doi:10.1093/humrep/dei011.
Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod BioMed Online. 2007;14(1):72–9.
Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, et al. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril. 2009;92(4):1306–11. doi:10.1016/j.fertnstert.2008.08.069.
Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod BioMed Online. 2009;19(2):171–80.
Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–95. doi:10.1016/j.fertnstert.2009.12.065.
Kazem R, Thompson LA, Srikantharajah A, Laing MA, Hamilton MP, Templeton A. Cryopreservation of human oocytes and fertilization by two techniques: in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1995;10(10):2650–4.
Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–6.
Palermo GD, Cohen J, Rosenwaks Z. Intracytoplasmic sperm injection: a powerful tool to overcome fertilization failure. Fertil Steril. 1996;65(5):899–908.
Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–6.
Parrott DM. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil. 1960;1:230–41.
Deanesly R. Immature rat ovaries grafted after freezing and thawing. J Endocrinol. 1954;11(2):197–200.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–91.
Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13(5):1133–8.
Oktay K, Karlikaya GG, Aydin BA. Ovarian cryopreservation and transplantation: basic aspects. Mol Cell Endocrinol. 2000;169(1–2):105–8.
Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. doi:10.1056/NEJM200006223422516.
Donnez J, Dolmans MM, Demylle D, Jadout P, Pirad C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10.
Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97 (2):387–90. doi:10.1016/j.fertnstert.2011.11.047.
Kolp LA, Hubayter Z. Autotransplantation of cryopreserved ovarian tissue: a procedure with promise, risks, and a need for a registry. Fertil Steril. 2011;95(6):1879–86. doi:10.1016/j.fertnstert.2011.02.049.
Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. doi:10.1016/S0140-6736(11)61781-9.
Ernst E, Kjaersgaard M, Birkebaek NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49(4):911–4. doi:10.1016/j.ejca.2012.09.028.
Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. doi:10.1093/molehr/gar082.
Resetkova N, Hayashi M, Kolp LA, Christianson MS. Fertility preservation for prepubertal girls: update and current challenges. Curr Obstet Gynecol Rep. 2013;2(4):218–25. doi:10.1007/s13669-013-0060-9.
Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1. doi:10.1093/humrep/deq033.
Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod. 2010;25(6):1590–1. doi:10.1093/humrep/deq096.
Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011a;95(5):1787 e1–4. doi:10.1016/j.fertnstert.2010.11.041.
Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–5. doi:10.1016/j.fertnstert.2012.05.017.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–21. doi:10.1056/NEJMc055237.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87(2):418 e7–e15. doi:10.1016/j.fertnstert.2006.05.086.
Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Med J Aust. 2013;198(3):158–9.
Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(1):268 e11–3. doi:10.1016/j.fertnstert.2009.09.046.
Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(7):2413 e15–9. doi:10.1016/j.fertnstert.2009.12.022.
Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99(1):227–30. doi:10.1016/j.fertnstert.2012.09.029.
Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008a;23(7):1531–7. doi:10.1093/humrep/den032.
Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94(3):324–8. doi:10.1111/aogs.12568.
Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod. 2014;29(8):1828. doi:10.1093/humrep/deu119.
Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–64. doi:10.1007/s10815-014-0331-z.
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011b;43(6):437–50. doi:10.3109/07853890.2010.546807.
Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103(2):462–8. doi:10.1016/j.fertnstert.2014.10.045.
Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–6. doi:10.1016/j.fertnstert.2009.12.073.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–15. doi:10.1093/humrep/deu353.
Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedders, Ernest E et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum reprod. 2015;30(12):2838–45. doi:10.1093/humrep/dev230.
Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–41. doi:10.1093/humrep/dew165.
Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33(12):1595–603. doi:10.1007/s10815-016-0814-1.
Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod. 2008;23(12):2709–17. doi:10.1093/humrep/den301.
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2016; doi:10.1007/s10815-016-0843-9.
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82(5):1390–4. doi:10.1016/j.fertnstert.2004.06.036.
Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–35. doi:10.1093/humupd/dml032.
Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87(4):971–5. doi:10.1016/j.fertnstert.2006.10.012.
Campbell BK, Hernandez-Medrano J, Onions V, Pincott-Allen C, Aljaser F, Fisher J, et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 2014;29(8):1749–63. doi:10.1093/humrep/deu144.
Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008b;359(24):2617–8. doi:10.1056/NEJMc0804321.
Mullen SF, Critser JK. The science of cryobiology. Cancer Treat Res. 2007;138:83–109.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–9. doi:10.1073/pnas.1312830110.
Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: case report. Hum Reprod. 1999;14(12):3077–9.
Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–70.
Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384(9950):1311–9. doi:10.1016/S0140-6736(14)61261-7.
Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–6.
Borini A, Bianchi V, Bonu MA, Sciajno R, Sereni E, Cattoli M, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod BioMed Online. 2007;15(2):175–81.
Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82(3):601–5. doi:10.1016/j.fertnstert.2004.04.025.
Bianchi V, Coticchio G, Distratis V, Di Giusto N, Flamigni C, Borini A. Differential sucrose concentration during dehydration (0.2 mol/l) and rehydration (0.3 mol/l) increases the implantation rate of frozen human oocytes. Reprod BioMed Online. 2007;14(1):64–71.
Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–6. doi:10.1016/j.fertnstert.2009.02.067.
Isachenko V, Isachenko E. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Cryo Letters. 2002;23(5):333–44.
Isachenko E, Isachenko V. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen Eur J Obstet Gynecol Reprod Biol. 2003;108(2):186–93.
Rahimi G, Isachenko E. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15(6):343–9.
Rahimi G, Isachenko E. Comparision of necrosis in human ovarian tissue after conventional slow freezing or vitrification. Reprod Biomed Online. 2004;9(2):187–93.
Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141(1):1–19. doi:10.1530/REP-10-0236.
Fahy GM. Vitrification: a new approach to organ cryopreservation. Prog Clin Biol Res. 1986;224:305–35.
Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–26.
Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;23(2):160–86. doi:10.1016/j.rbmo.2011.04.005.
Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod BioMed Online. 2005;11(5):608–14.
Sanfilippo S, Canis M, Smitz J, Sion B, Darcha C, Janny L, et al. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing. Reprod Biol Endocrinol. 2015;13:67. doi:10.1186/s12958-015-0065-5.
Sonmezer M, Oktay K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):113–26. doi:10.1016/j.bpobgyn.2009.09.002.
Soares M, Dolmans MM, Donnez J. Heterotopic ovarian tissue transplantation. In: Suzuki N, Donnez J, editors. Gonadal tissue cryopreservation in fertility preservation. Japan: Springer. 2016. pp. 105–23.
Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95(2):695–701. doi:10.1016/j.fertnstert.2010.07.1080.
Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21(6):1345–8. doi:10.1093/humrep/del007.
Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril. 2011;95(2):804 e7–10. doi:10.1016/j.fertnstert.2010.07.1072.
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–65. doi:10.1093/humupd/dmp021.
Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–97. doi:10.1016/j.bpobgyn.2014.09.003.
Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–31. doi:10.1007/s10815-015-0528-9.
Fasano G, Moffa F, Dechene J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150. doi:10.1186/1477-7827-9-150.
Park CW, Lee SH, Yang KM, Lee IH, Lim KT, Lee KH, et al. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin Exp Reprod Med. 2016;43(2):119–25. doi:10.5653/cerm.2016.43.2.119.
Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33(6):741–6. doi:10.1007/s10815-016-0691-7.
Revel A, Revel-Vilk S, Aizenman E, Porat-Katz A, Safran A, Ben-Meir A, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92(2):458–63. doi:10.1016/j.fertnstert.2008.07.013.
Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–7. doi:10.1016/j.fertnstert.2010.06.064.
Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31(4):750–62. doi:10.1093/humrep/dew007.
Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13. doi:10.1093/humrep/den055.
Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25(7):1708–12. doi:10.1093/humrep/deq121.
Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–8. doi:10.1093/humrep/det420.
Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts Views Vis Obgyn. 2014;6(2):77–80.
Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104(5):1258–60. doi:10.1016/j.fertnstert.2015.07.1148.
Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009a;91(2):372–6. doi:10.1016/j.fertnstert.2007.11.088.
Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009b;91(6):2391–8. doi:10.1016/j.fertnstert.2008.04.014.
Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89(3):567–72. doi:10.1016/j.fertnstert.2007.03.090.
Hourvitz A, Yerushalmi GM, Maman E, Raanani H, Elizur S, Brengauz M, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31(4):497–505. doi:10.1016/j.rbmo.2015.06.025.
Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. doi:10.1210/er.2014-1020,10.1210/er.2015.36.issue-1.edboard.
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107(22):10280–4. doi:10.1073/pnas.1001198107.
Cordeiro CN, Christianson MS, Selter JH, Segars Jr JH. In vitro activation: a possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci. 2016;23(4):429–38. doi:10.1177/1933719115625842.
Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol. 2016;28(3):217–22. doi:10.1097/GCO.0000000000000268.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–92.
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. doi:10.1371/journal.pone.0019475.
Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17(23–24):3095–104. doi:10.1089/ten.TEA.2011.0204.
Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94 e1–9. doi:10.1016/j.ajog.2015.10.001.
Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24(11):2778–87. doi:10.1093/humrep/dep289.
Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30(1):11–24. doi:10.1007/s10815-012-9912-x.
Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sorensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120(22):4311–6. doi:10.1182/blood-2012-01-403022.
Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67(1):64–9. doi:10.1016/j.cryobiol.2013.05.002.
O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–6. doi:10.1095/biolreprod.102.013029.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–8. doi:10.1093/humrep/den070.
Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27(6):1801–10. doi:10.1093/humrep/der468.
Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30(10):1279–88. doi:10.1007/s10815-013-0052-8.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–72. doi:10.1093/humrep/den244.
Mol BW, Zoll M. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):507. doi:10.1016/S0140-6736(15)60199-4.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):506–7. doi:10.1016/S0140-6736(15)60198-2.
Stoop D, Silber S, Cobo A. Fertility preservation for age-related fertility decline—authors’ reply. Lancet. 2015;385(9967):507–8. doi:10.1016/S0140-6736(15)60200-8.
Acknowledgements
This work was supported by the Howard W. and Georgeanna Seegar Jones Endowment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ladanyi, C., Mor, A., Christianson, M.S. et al. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J Assist Reprod Genet 34, 709–722 (2017). https://doi.org/10.1007/s10815-017-0899-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0899-1