Abstract
Purpose
The study aims to investigate the genetic association between paired box gene 2 (PAX2) and mullerian duct anomalies (MDA) in Chinese Han females.
Methods
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used to identify the genotypes of three tag single nucleotide polymorphisms (SNPs) in PAX2 in 362 MDA cases and 406 controls.
Results
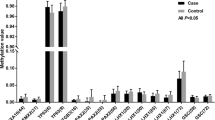
We found that one tag SNP (rs12266644) of PAX2 was associated with susceptibility to MDA. The genotype distributions of the SNP rs12266644 have a statistically significant difference in the MDA patients and controls with a p value = 0.008. In the dominant model, we also observed that the GT + TT genotype increased the risk for MDA (p = 0.015, OR = 1.637, 95 % CI = 1.096–2.443).
Conclusion
The polymorphism rs12266644 of PAX2 might be a risk factor for MDA in Chinese Han females.

Similar content being viewed by others
References
The American Fertility Society classifications of adnexal adhesions. Distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–55.
Ribeiro SC, Tormena RA, Peterson TV, et al. Mullerian duct anomalies: review of current management. Sao Paulo Med J Rev Paul Med. 2009;127(2):92–6.
Vallerie AM, Breech LL. Update in Mullerian anomalies: diagnosis, management, and outcomes. Curr Opin Obstet Gynecol. 2010;22(5):381–7.
Acien P, Acien M, Sanchez-Ferrer M. Complex malformations of the female genital tract. New types and revision of classification. Hum Reprod. 2004;19(10):2377–84.
Pittock ST, Babovic-Vuksanovic D, Lteif A. Mayer-Rokitansky-Kuster-Hauser anomaly and its associated malformations. Am J Med Genet A. 2005;135(3):314–6.
Cheroki C, Krepischi-Santos AC, Szuhai K, et al. Genomic imbalances associated with mullerian aplasia. J Med Genet. 2008;45(4):228–32.
Philibert P, Biason-Lauber A, Gueorguieva I, et al. Molecular analysis of WNT4 gene in four adolescent girls with mullerian duct abnormality and hyperandrogenism (atypical Mayer-Rokitansky-Kuster-Hauser syndrome). Fertil Steril. 2011;95(8):2683–6.
Wu K, Chang X, Wei D, Xu C, Qin Y, Chen ZJ. Lack of association of WNT5A mutations with Mullerian duct abnormalities. Reprod Biomed Online. 2013;26(2):164–7.
Dang Y, Qin Y, Tang R, et al. Variants of the WNT7A gene in Chinese patients with mullerian duct abnormalities. Fertil Steril. 2012;97(2):391–4.
Tang R, Dang Y, Qin Y, et al. WNT9B in 542 Chinese women with Mullerian duct abnormalities: mutation analysis. Reprod Biomed Online. 2014;28(4):503–7.
Chen X, Mu Y, Li C, et al. Mutation screening of HOXA7 and HOXA9 genes in Chinese women with Mullerian duct abnormalities. Reprod Biomed Online. 2014;29(5):595–9.
Ekici AB, Strissel PL, Oppelt PG, et al. HOXA10 and HOXA13 sequence variations in human female genital malformations including congenital absence of the uterus and vagina. Gene. 2013;518(2):267–72.
Chen X, Li G, Qin Y, Cui Y, You L, Chen ZJ. Mutations in HOXA11 are not responsible for Mullerian duct anomalies in Chinese patients. Reprod Biomed Online. 2014;28(6):739–42.
Sandbacka M, Laivuori H, Freitas E, et al. TBX6, LHX1 and copy number variations in the complex genetics of Mullerian aplasia. Orphanet J Rare Dis. 2013;8:125.
Kuschert S, Rowitch DH, Haenig B, McMahon AP, Kispert A. Characterization of Pax-2 regulatory sequences that direct transgene expression in the Wolffian duct and its derivatives. Dev Biol. 2001;229(1):128–40.
Tong G-X, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Müllerian system origin? Mod Pathol. 2007;20(8):856–63.
Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Dev (Cambridge, England). 1995;121(12):4057–65.
Favor J, Sandulache R, Neuhauser-Klaus A, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci U S A. 1996;93(24):13870–5.
Schimmenti LA. Renal coloboma syndrome. Eur J Human Genet: EJHG. 2011;19(12):1207–12.
Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169(4):505–14.
YongYongSHI LHE. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97–8.
Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4(12):969–80.
Rackow BW, Arici A. Reproductive performance of women with mullerian anomalies. Curr Opin Obstet Gynecol. 2007;19(3):229–37.
Gruss P, Walther C. Pax in development. Cell. 1992;69(5):719–22.
Sharma R, Sanchez-Ferras O, Bouchard M. Pax genes in renal development, disease and regeneration. Semin Cell Dev Biol. 2015;44:97–106.
Rabban JT, McAlhany S, Lerwill MF, Grenert JP, Zaloudek CJ. PAX2 distinguishes benign mesonephric and mullerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. 2010;34(2):137–46.
Klattig J, Englert C. The Mullerian duct: recent insights into its development and regression. Sex Dev: Genet, Mol Biol, Evol, Endocrinol, Embryol, Pathol Sex Determ Differ. 2007;1(5):271–8.
Eberhard D, Jimenez G, Heavey B, Busslinger M. Transcriptional repression by Pax5 (BSAP) through interaction with corepressors of the Groucho family. EMBO J. 2000;19(10):2292–303.
Cai Y, Lechner MS, Nihalani D, Prindle MJ, Holzman LB, Dressler GR. Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J Biol Chem. 2002;277(2):1217–22.
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–92.
Huang CC, Orvis GD, Kwan KM, Behringer RR. Lhx1 is required in Mullerian duct epithelium for uterine development. Dev Biol. 2014;389(2):124–36.
Acknowledgments
The authors thank all the participants in this study; without them, the research could not have been performed. This study was supported by the National Natural Science Foundation of China (81370691), the National Basic Research Program of China (2012CB944704), and the Research Fund of National Health and Family Planning Commission of China (201402004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All the participants signed written informed consents. Our experiment was approved by the local ethics committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Capsule The PAX2 variant rs12266644 might increase the risk for mullerian duct anomalies in Chinese women.
Zuying Xu and Shinan Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, Z., Wu, S., Xing, Q. et al. Genetic association between PAX2 and mullerian duct anomalies in Han Chinese females. J Assist Reprod Genet 34, 125–129 (2017). https://doi.org/10.1007/s10815-016-0807-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0807-0