Abstract
Purpose
To investigate the usefulness of preimplantation genetic diagnosis (PGD) for the patient affected by congenital contractural arachnodactyly (CCA) and spinal and bulbar muscular atrophy (SBMA).
Methods
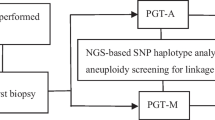
Multiple displacement amplification (MDA) was performed for whole genome amplification (WGA) of biopsied trophectoderm (TE) cells. Direct mutation detection by sequencing and next-generation sequencing (NGS)-based single nucleotide polymorphism (SNP) haplotyping were used for CCA diagnosis. Direct sequencing of the PCR products and sex determination by amplification of sex-determining region Y (SRY) gene were used for SBMA diagnosis. After PGD, the unaffected blastocyst (B4) was transferred in the following frozen embryo transfer (FET).
Results
In this PGD cycle, sixteen MII oocytes were inseminated by ICSI with testicular spermatozoa. Four blastocysts (B4, B5, B10, B13) were utilized for TE cell biopsy on day 5 after ICSI. After PGD, B4 was unaffected by CCA and SBMA. B5 was affected by CCA and carried SBMA. B10 was unaffected by CCA and carried SBMA. B13 was affected by CCA and unaffected by SBMA. B4 was the only unaffected blastocyst and transferred into the uterus for the subsequent FET cycle. The accuracy of PGD was confirmed by amniocentesis at 21 weeks of gestation. A healthy boy weighing 2850 g was born by cesarean section at the 38th week of gestation.
Conclusions
PGD is a valid screening tool for patienst affected of CCA and SBMA to prevent transmission of these genetic diseases from parents to children.





Similar content being viewed by others
References
Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344:768–70.
De Rycke M, Belva F, Goossens V, Moutou C, SenGupta SB, Traeger-Synodinos J, et al. ESHRE PGD Consortium data collection XIII: cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum Reprod. 2015;30:1763–89.
Eldar-Geva T, Srebnik N, Altarescu G, Varshaver I, Brooks B, Levy-Lahad E, et al. Neonatal outcome after preimplantation genetic diagnosis. Fertil Steril. 2014;102:1016–21.
Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, et al. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod. 2012;27:288–93.
Jurko Jr A, Krsiakova J, Minarik M, Tonhajzerova I. Congenital contractural arachnodactyly (Beals-Hecht syndrome): a rare connective tissue disorder. Wien Klin Wochenschr. 2013;125:288–90.
Tunçbilek E, Alanay Y. Congenital contractural arachnodactyly (Beals syndrome). Orphanet J Rare Dis. 2006;1:20–2.
Liu W, Zhao N, Li XF, Wang H, Sui Y, Lu YP, et al. A novel FBN2 mutation in a Chinese family with congenital contractural arachnodactyly. FEBS Open Bio. 2015;5:163–6.
Frédéric MY, Monino C, Marschall C, Hamroun D, Faivre L, Jondeau G, et al. The FBN2 gene: new mutations, locus-specific database (Universal Mutation Database FBN2), and genotype-phenotype correlations. Hum Mutat. 2009;30:181–90.
Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995;11:456–8.
Gupta PA, Wallis DD, Chin TO, Northrup H, Tran-Fadulu VT, Towbin JA, et al. FBN2 mutation associated with manifestations of Marfan syndrome and congenital contractural arachnodactyly. J Med Genet. 2004;41:e56–8.
Inbar-Feigenberg M, Meirowitz N, Nanda D, Toi A, Okun N, Chitayat D. Beals syndrome (congenital contractural arachnodactyly): prenatal ultrasound findings and molecular analysis. Ultrasound Obstet Gynecol. 2014;44:486–90.
Kölble N, Wisser J, Babcock D, Maslen C, Huch R, Steinmann B. Prenatal ultrasound findings in a fetus with congenital contractural arachnodactyly. Ultrasound Obstet Gynecol. 2002;20:395–9.
Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–80.
Grunseich C, Kats IR, Bott LC, Rinaldi C, Kokkinis A, Fox D, et al. Early onset and novel features in a spinal and bulbar muscular atrophy patient with a 68 CAG repeat. Neuromuscul Disord. 2014;24:978–81.
Sinclair R, Greenland KJ, Egmond S, Hoedemaker C, Chapman A, Zajac JD. Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol. 2007;157:290–4.
Li M, Miwa S, Kobayashi Y, Merry DE, Yamamoto M, Tanaka F, et al. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol. 1998;44:249–54.
Wilton L, Thornhill A, Traeger-Synodinos J, Sermon KD, Harper JC. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;24:1221–8.
Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, SenGupta SB, et al. The ESHRE PGD Consortium: 10 years of data collection. Hum Reprod Update. 2012;18:234–47.
Kokkali G, Traeger-Synodinos J, Vrettou C, Stavrou D, Jones GM, Cram DS, et al. Blastocyst biopsy versus cleavage stage biopsy and blastocyst transfer for preimplantation genetic diagnosis of beta-thalassaemia: a pilot study. Hum Reprod. 2007;22:1443–9.
Renwick PJ, Trussler J, Ostad-Saffari E, Fassihi H, Black C, Braude P, et al. Proof of principle and first cases using preimplantation genetic haplotyping—a paradigm shift for embryo diagnosis. Reprod Biomed Online. 2006;13:110–9.
Qubbaj W, Al-Swaid A, Al-Hassan S, Awartani K, Deek H, Coskun S. First successful application of preimplantation genetic diagnosis and haplotyping for congenital hyperinsulinism. Reprod Biomed Online. 2011;22:72–9.
Renwick P, Trussler J, Lashwood A, Braude P, Ogilvie CM. Preimplantation genetic haplotyping: 127 diagnostic cycles demonstrating a robust, efficient alternative to direct mutation testing on single cells. Reprod Biomed Online. 2010;20:470–6.
Chang LJ, Huang CC, Tsai YY, Hung CC, Fang MY, Lin YC, et al. Blastocyst biopsy and vitrification are effective for preimplantation genetic diagnosis of monogenic diseases. Hum Reprod. 2013;28:1435–44.
Chen YL, Hung CC, Lin SY, Fang MY, Tsai YY, Chang LJ, et al. Successful application of the strategy of blastocyst biopsy, vitrification, whole genome amplification, and thawed embryo transfer for preimplantation genetic diagnosis of neurofibromatosis type 1. Taiwan J Obstet Gynecol. 2011;50:74–8.
Altarescu G, Zeevi DA, Zeligson S, Perlberg S, Eldar-Geva T, Margalioth EJ, et al. Familial haplotyping and embryo analysis for preimplantation genetic diagnosis (PGD) using DNA microarrays: a proof of principle study. J Assist Reprod Genet. 2013;30:1595–603.
Altarescu G, Eldar Geva T, Brooks B, Margalioth E, Levy-Lahad E, Renbaum P. PGD on a recombinant allele: crossover between the TSC2 gene and ‘linked’ markers impairs accurate diagnosis. Prenat Diagn. 2008;28:929–33.
Kakourou G, Dhanjal S, Daphnis D, Doshi A, Nuttall S, Gotts S, et al. Preimplantation genetic diagnosis for myotonic dystrophy type 1: detection of crossover between the gene and the linked marker APOC2. Prenat Diagn. 2007;27:111–6.
Lewis, R. Human genetics: concepts and applications. McGraw-Hill Higher Education; 2005
La Spada AR, Roling DB, Harding AE, Warner CL, Spiegel R, Hausmanowa-Petrusewicz I, et al. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992;2:301–4.
Dossena M, Bedini G, Rusmini P, Giorgetti E, Canazza A, Tosetti V, et al. Human adipose-derived mesenchymal stem cells as a new model of spinal and bulbar muscular atrophy. PLoS One. 2014;9, e112746.
Georgiou I, Sermon K, Lissens W, De Vos A, Platteau P, Lolis D, et al. Preimplantation genetic diagnosis for spinal and bulbar muscular atrophy (SBMA). Hum Genet. 2001;108:494–8.
Rhodes LE, Freeman BK, Auh S, Kokkinis AD, La Pean A, Chen C, et al. Clinical features of spinal and bulbar muscular atrophy. Brain. 2009;132:3242–51.
Acknowledgments
We thank the family for their participation in this study. We are extremely grateful to Peking Jabrehoo Med Tech., Ltd for this work. This work was funded by Medical Science and technology development Foundation, Nanjing Department of Health (YKK15070), and the special grant for clinical medicine science of Jiangsu Province (BL2014003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The couple signed informed consent forms for ICSI treatment, PGD, and follow-up.
This project was approved by the Ethics Committee of the Drum Tower Hospital, which is affiliated to Nanjing University Medical College.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The URLs for data in this article are as follows:
OMIM, http://www.ncbi.nlm.nih.gov/omim/
1000 Genomes, http://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/
Primers design for PGH, https://www.ampliseq.com/
Capsule
A description of a PGD cycle for the diagnosis of CCA and SBMA, resulting in the birth of a healthy boy.
Linjun Chen and Zhenyu Diao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, L., Diao, Z., Xu, Z. et al. The clinical application of preimplantation genetic diagnosis for the patient affected by congenital contractural arachnodactyly and spinal and bulbar muscular atrophy. J Assist Reprod Genet 33, 1459–1466 (2016). https://doi.org/10.1007/s10815-016-0760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0760-y