Abstract
Purpose
The purpose of this study is to investigate if female patients with lymphoma demonstrate diminished ovarian reserve prior to initiation of the lymphoma treatment.
Methods
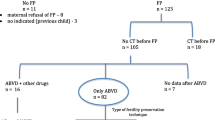
Sixty-four patients with newly diagnosed lymphoma undergoing controlled ovarian hyperstimulation for fertility preservation were compared with 365 healthy controls undergoing elective oocyte cryopreservation (controlled ovarian hyperstimulation (COH)) and 128 patients with other types of malignancy prompting fertility preservation. The data of all lymphoma patients, all elective, and all the patients with other types of malignancy who met the inclusion criteria and underwent COH for fertility preservation during the study period were retrospectively analyzed. Primary outcomes included serum anti-Müllerian hormone (AMH) levels (ng/mL) and antral follicle count (AFC).
Results
Patients in the lymphoma group demonstrated significantly lower AMH levels and AFC and had less oocytes harvested and cryopreserved when compared to healthy controls as well as patients with other malignancies.
Conclusion
Patients with lymphoma demonstrate diminished ovarian reserve when compared with healthy controls and patients with other malignancies. This should be taken into consideration when deciding on the dose for COH.
Similar content being viewed by others
References
U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 incidence and mortality Web-based report. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. Available at: www.cdc.gov/uscs.
Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011;66(4):248–54.
Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–12.
Jadoul P, Kim SS, ISFP Practice Committee. Fertility considerations in young women with hematological malignancies. J Assist Reprod Genet. 2012;29(6):479–87.
Viviani S, Ragni G, Santoro A, Perotti L, Caccamo E, Negretti E, et al. Testicular dysfunction in Hodgkin's disease before and after treatment. Eur J Cancer. 1991;27(11):1389–92.
Rueffer U, Breuer K, Josting A, Lathan B, Sieber M, Manzke O, et al. Male gonadal dysfunction in patients with Hodgkin's disease prior to treatment. Ann Oncol. 2001;12:1307–11.
Sklavos MM, Stratton P, Giri N, Alter BP, Savage SA, Pinto LP. Reduced serum levels of anti-Müllerian hormone in females with inherited bone marrow failure syndromes. J Clin Endocrinol Metabol. 2015;100:E197–203.
Quintero RB et al. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93:865–8.
Johnson LN et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013;26:337–44.
Davis OK, Rosenwaks Z. Superovulation strategies for assisted reproductive technologies, 2001.19:207-12.
Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98(1):141–4.
Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280–5.
Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–9.
Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's lymphoma study group. J Clin Oncol. 2005;23:7555–64.
Johnson LN, Sammel MD, Dillon KE, Lechtenberg L, Schanne A, Gracia CR. Antimüllerian hormone and antral follicle count are lower in female cancer survivors and healthy women taking hormonal contraception. Fertil Steril. 2014;102:774–81.
Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9.
Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-Müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650–5.
Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003–10.
Checa MA, Brassesco M, Sastre M, et al. Random-start GnRH antagonist for emergency fertility preservation: a self-controlled trial. Int J Womens Health. 2015;7:219–25.
Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–11.
Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril. 2011;96(1):e51–4.
Ozkaya E, San Roman G, Oktay K. Luteal phase GnRHa trigger in random start fertility preservation cycles. J Assist Reprod Genet. 2012;29(6):503–5.
Moore JA, Moore MB, Samaniego F, Pinnix CC, Moore Jr DF. Small lymphocytic lymphoma presenting with hypopituitarism. Am J Med. 2016;129(1):e9–e10.
Valeros KA, Khoo E. Anterior panhypopituitarism in diffuse large B-cell stage IV lymphoma. J Clin Neurosci. 2014;21(8):1464–6.
Schleinitz N, Bernit E, Mazodier K, Charbonnier A, Horchowski N, Andrac-Meyer L, et al. Two cases of intravascular lymphomatosis disclosing with hypopituitarism. Haematologica. 2002;87(6):ECR21.
Mourand I, Menjot de Champfleur N, Bauchet L, Dumontel T, Corlobé A, Quittet P, et al. Reversible hypothalamic-pituitary axis involvement in a patient with intravascular lymphomatosis. J Neuroradiol. 2014;41(5):360–2.
Shapira M, Raanani H, Meirow D. IVF for fertility preservation in breast cancer patients—efficacy and safety issues. J Assist Reprod Genet. 2015 Jul 1.
Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5.
Kim JH et al. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci. 2015;30:290–5.
Revelli A et al. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol. 2013;29:993–6.
Johnson J, Canning J, Keneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50.
Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15.
Hill JA, Haimovici D, Politich JA, Anderson DJ. Effects of soluble products of activated lymphocytes on macrophages (lymphokines and monokines) on human sperm motion parameters. Fertil Steril. 1987;47:460–5.
Hu R, Miao M, Zhang R, Li Y, Li J, Zhu K, et al. Ovary involvement of diffuse large B cell lymphoma. Am J Case Rep. 2012;13:96–8.
Chorlton I, Norris HJ, King FM. Malignant reticuloendothelial disease involving the ovary as a primary manifestation: a series of 19 lymphomas and 1 granulocytic sarcoma. Cancer. 1974;34(2):397–407.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Weill Cornell Medical College institutional review board approved this study.
Conflict of interest
The authors declare that they have no competing interests.
Financial support
Institutional
Additional information
Capsule Female patients with lymphoma demonstrate diminished ovarian reserve when compared with healthy controls as well as patients with other type of malignancy even before initiation of chemotherapy.
Rights and permissions
About this article
Cite this article
Lekovich, J., Lobel, A.L.S., Stewart, J.D. et al. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet 33, 657–662 (2016). https://doi.org/10.1007/s10815-016-0689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0689-1