Abstract
Purpose
This study investigated the prevalence of abnormally cleaved embryos and determined which types of abnormally cleaved embryos (1-3c, 2-4c, 3-5c, 4-6c), might be suitable for transfer based on live birth data.
Methods
One hundred seventy-one women (whose transferred embryos were confirmed to be either fully implanted or fully unimplanted) provided 1256 embryos, which were analyzed.
Results
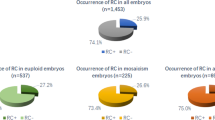
Of these embryos, 320 embryos were transferred, of these transferred embryos, 291 embryos were normal and 29 embryos were abnormal, which five embryos were not analyzed because each one was presented one abnormal cleavage type. These 24 embryos were divided into four groups. Inclusion criteria were as follows: women under 37 years of age undergoing first fresh in vitro fertilization (IVF) treatment with a basal antral follicle count of 5–15, body mass index (BMI) of 18–25 kg/m2, number of retrieved oocytes between 5 and 20, and tubal factors as the cause of infertility. Time-lapse imaging analysis software was used to compare temporal parameters of normal cleavage and abnormal cleavage groups (there were four abnormal groups, based on the prevalence of abnormal cleavage embryos). Cleavage times were analyzed before the abnormal cleavage occurred, and time intervals were analyzed after the abnormal cleavage based upon the types of abnormal cleavage. In addition, the time intervals of t4-t3 and t8-t5 were also analyzed; corresponding time parameters were measured in the normal group as well. Implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate were also measured in the normally cleaved and abnormally cleaved embryos. The prevalence of abnormal cleavage was 15.92 % (200/1256). T8-t5 was the most important parameter in the prediction of potential development (production of a live-born baby) of abnormally cleaving embryos.
Conclusions
Abnormally cleaving embryos were able to produced live births with T8-t5 the best parameter to predict the developmental potential of abnormally cleaving embryos.


Similar content being viewed by others
References
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. Fertil Steril. 2010;94:1152–3.
Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–40.
Gardner DK, Sakkas D. Assessment of embryo viability: the ability to select a single embryo for transfer—a review. Placenta. 2003;24:S5–S12.
Hlinka D, Kalatova B, Uhrinova I, Dolinska S, Rutarova J, Rezacova J, et al. Time-lapse cleavage rating predicts human embryo viability. Physiol Res. 2012;61:513–25.
Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–9.
Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–4.
Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–85.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12:532–41.
Wirka KA, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48.
Dale B, Elder K. In vitro fertilization. United Kingdom: Cambrige University; 1997. p. 115–6.
Ye H, Huang GN, Zeng PH, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. J Assist Reprod Genet. 2009;26:105–11.
Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Yang ST, Shi JX, Gong F, Zhang SP, Lu CF, Tan K, et al. Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod BioMed Online. 2015;2:1–9.
Yu SL, Lee RK, Su JT, Chih YF, Tsai YC, Lin MH, et al. Distinction between paternal and maternal contributions to the tripronucleus in the human zygotes obtained after in vitro fertilization. Taiwan J Obstet Gynecol. 2006;45:313–6.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37:395–401.
Kalatova B, Jesenska R, Hlinka D, Dudas M. Tripolar mitosis in human cells and embryos: occurrence, pathophysiology and medical implications. Acta Histochem. 2015;117:111–25.
Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L, et al. Cytogenetic results from the US collaborative study on CVS. Prenat Diagn. 1992;12:317–45.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update 2014. doi:10.1093/humupd/dmu016.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod BioMed Online. 2013;27:140–6.
Herrero J, Tejera A, Albert C, Vidal C, de los Santos MJ, Meseguer M. A time to look back: analysis of morphokinetic characteristics of human embryo development. Fertil Steril. 2013;100:1602–9.
Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12:54.
Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30:703–10.
Acknowledgments
The authors gratefully acknowledge Lihong Wu for her technical support regarding the management of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures and protocols were approved by the ethics committee of the Chongqing Obstetric and Gynecology Hospital ethical number RGIEA-201405.
Additional information
Capsule Abnormally cleaving embryos were able to produced live births with T8-t5 the best parameter to predict the developmental potential of abnormally cleaving embryos.
Rights and permissions
About this article
Cite this article
Fan, Y.L., Han, S.B., Wu, L.H. et al. Abnormally cleaving embryos are able to produce live births: a time-lapse study. J Assist Reprod Genet 33, 379–385 (2016). https://doi.org/10.1007/s10815-015-0632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0632-x