Abstract
Purpose
This is a retrospective analysis of the morphokinetics, prevalence, and implantation potential of embryos with irregular first and second cleavages as identified by time-lapse microscopy.
Methods
The study included 253 women who underwent 387 assisted reproduction treatments with intracytoplasmic sperm injection (ICSI). Each patient was assigned to one of three groups based on embryo cleavage results. In group I, one to two embryos per cycle showed irregular cleavage; group II, at least three embryos with abnormal cleavage; and in group III (the control group), all embryos cleaved normally. The number of embryos that cleaved from 1 to ≥3 cells or from 2 to ≥5 cells for each patient was recorded. Their prevalence and association with women’s characteristics and pregnancy outcome were evaluated.
Results
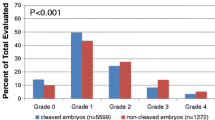
The prevalence of irregular cleavage was 15.6 % among 1772 ICSI embryos. In 101 cycles, 1–2 embryos per cycle showed irregular cleavage (group I). In 32 cycles, at least 3 embryos showed abnormal cleavage (group II). In 254 cycles, all embryos cleaved normally (group III). The average age of the women in group II was significantly lower in comparison with groups I and III (32.5 ± 4.2 vs. 35.1 ± 4.9 and 35.5 ± 5.1, respectively, p < 0.02). In comparison of groups I and II, the odds ratio for ≥3 embryos with irregular cleavage in women younger than 35 was 3.48 (95 % CI, 1.28 to 9.46). Embryos with irregular cleavage were transferred in 16 women. Three live births were achieved following the transfer of single blastocysts derived from embryos with irregular cleavage from two to five cells.
Conclusions
Early embryos with irregular cleavage are significantly more prevalent in younger women. When these embryos develop to the blastocyst stage, they may have normal implantation potential, leading to the birth of healthy babies.
Similar content being viewed by others
References
Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–85.
Chen AA, Tan I, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation and practice application. Fertil Steril. 2013;99:1035–43.
Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–34.
Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98:1481–9.
Wirka KA, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Yang ST, Shi JX, Gong F, Zhang SP, Lu CF, Tan K, et al. Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod Biomed Online. 2015;30:625–34.
Somfai T, Inaba Y, Aikawa Y, Ohtake M, Kobayashi S, Konishi K, et al. Relationship between the length of cell cycles, cleavage pattern and developmental competence in bovine embryos generated by IVF or parthenogenesis. J Reprod Dev. 2010;56:200–7.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage pattern and subsequent kariotypic analysis of embryos. Biol Reprod. 1987;37:395–401.
Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, et al. Dynamic blastomere behavior reflects human embryo ploidy by the four cell stage. Nat Commun. 2012;2249:1–12.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;4:571–81.
Wells D, Delhanty JD. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod. 2000;6:1055–62.
Mertzanidou A, Wilton L, Cheng J, Spits C, Vanneste E, Moreau Y, et al. Microarray analysis reveals abnormal chromosome complements in over 70% of 14 normally developing human embryos. Hum Reprod. 2013;28:256–64.
Chow JFC, Yeung WSB, Lau EYL, Lee VCY, Ng EHY, Ho PC. Array comparative genomic hybridization analyses of all blastomeres of a cohort of embryos from young IVF patients revealed significant contribution of mitotic errors to embryo mosaicism at the cleavage stage. Reprod Biol Endocrinol. 2014;12:105–14.
Stecher A, Vanderzwalmen P, Zintz M, Wirleitner B, Schuff M, Spitzer D, et al. Transfer of blastocysts with deviant morphological and morphokinetic parameters at early stages of in vitro development: a case series. Reprod Biomed Online. 2014;28:424–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Study approval was obtained from the Kaplan Medical Center Institutional Review Board.
Additional information
Capsule
Prevalence of early embryos with irregular cleavage was significantly higher in younger women, and transfer of these embryos that reach the blastocyst stage may result in the birth of healthy babies.
Rights and permissions
About this article
Cite this article
Almagor, M., Or, Y., Fieldust, S. et al. Irregular cleavage of early preimplantation human embryos: characteristics of patients and pregnancy outcomes. J Assist Reprod Genet 32, 1811–1815 (2015). https://doi.org/10.1007/s10815-015-0591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0591-2