Abstract
Purpose
This study aimed to evaluate whether a gelatin-based Gelfoam sponge is feasible as a scaffold for adipose tissue-derived stem cell (ASC) therapy in rat frozen-thawed ovarian autografts.
Methods
Two sets of studies were performed. The in vitro set evaluated ASCs’ viability in the Gelfoam scaffold at different times of co-culturing (after 24, 48, 72, 96, and 120 h). The in vivo set used 20 12-week-old adult female Wistar rats. Frozen-thawed ovarian grafts were treated with ASCs delivered in Gelfoam scaffolds immediately after an autologous retroperitoneal transplant (ASCs-GS, n = 10). The controls received Gelfoam with a culture medium (GS, n = 10). Assessment of graft quality was conducted by vaginal smears (until euthanasia on the 30th postoperative day), histological analyses, follicular density, and viability and fibrosis. Immunohistochemical staining for VEGF-A expression, vascular network (vWF), apoptosis (caspase-3 and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)), cell proliferation (Ki-67), and hormone receptors (estrogen and progesterone) were performed.
Results
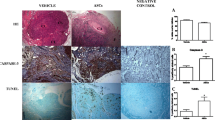
The cells remained viable in Gelfoam for up to 120 h of co-culturing. The graft morphology was similar among the groups. ASC therapy promoted the earlier resumption of the estrous phase (GS 16.6 ± 3 vs. ASCs-GS 12.8 ± 1.3 days) and enhanced estrogen receptors compared with the controls (p < 0.05) without interfering with the quantity and viability of the ovarian follicles, fibrosis, endothelial cells, VEGF immunoexpression, apoptosis, or cell proliferation (p > 0.05).
Conclusion
The Gelfoam scaffold could be a feasible and safe non-invasive technique for ASC delivery in the treatment of frozen-thawed ovarian autografts. Future studies should evaluate the real benefit of this treatment on the survival and endocrine activity of the graft.




Similar content being viewed by others
References
The Practice Committee of the American Society for Reproductive Medicine. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–43.
Lunardi FO, Araújo VR, Bernuci MP, Lunardi LO, Gonçalves RF, Carvalho Ade A, et al. Restoring fertility after ovarian tissue cryopreservation: a half century of research. Zygote. 2013;21(4):394–405.
Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2012;97(6):1260–8.
Szöke K, Brinchmann JE. Concise review: therapeutic potential of adipose tissue-derived angiogenic cells. Stem Cells Transl Med. 2012;1:658–67.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60.
Cherubino M, Rubin JP, Miljkovic N, Kelmendi-Doko A, Marra KG. Adipose-derived stem cells for wound healing applications. Ann Plast Surg. 2011;66(2):210–5.
Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10:353–63.
Sun M, Wang S, Yu L, Gu F, Wang C, Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4:80.
Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93:181–93.
Damous LL, Nakamuta JS, Teofilo de Carvalho AE, Soares-Jr JM, Simões MJ, Krieger JE, Baracat EC. Adipose tissue-derived stem cells in cryopreserved ovarian grafts. Stem Cell Res Ther. 2015;6(1):57. Epub ahead of print.
Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17(23–24):3095–104.
Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–82.
Gao JM, Yan J, Li R, Li M, Yan LY, Wang TR, et al. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum Reprod. 2013;28(10):2784–93.
Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, et al. Transplantation of an alginate-Matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–85.
Cha SK, Shin DH, Kim BY, Yoon SY, Yoon TK, Lee WS, et al. Effect of human endothelial progenitor cell (EPC)- or mouse vascular endothelial growth factor-derived vessel formation on the survival of vitrified/warmed mouse ovarian grafts. Reprod Sci. 2014;21(7):859–68.
Henry L, Labied S, Fransolet M, Kirschvink N, Blacher S, Noel A, et al. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularisation after xenotransplantation in mice. Reprod Biol Endocrinol. 2015;13(1):15.
Amoh Y, Li L, Katsuoka K, Bouvet M, Hoffman R. GFP-expressing vascularization of Gelfoam® as a rapid in vivo assay of angiogenesis stimulators and inhibitors. BioTechniques. 2007;42:294–8.
Methe H, Nugent HM, Groothuis A, Seifert P, Sayegh MH, Edelman ER, et al. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112:I–I-95.
Lee JY, Choi MH, Shin EY, Kang YK. Autologous mesenchymal stem cells loaded in Gelfoam(®) for structural bone allograft healing in rabbits. Cell Tissue Bank. 2011;12(4):299–309.
Mii S, Uehara F, Yano S, Tran B, Miwa S, Hiroshima Y, et al. Nestin-expressing stem cells promote nerve growth in long-term 3-dimensional Gelfoam®-supported histoculture. Plos One. 2013;8(6), e67153.
Nugent MH, Sjin RTT, White D, Milton LG, Manson RJ, Lawson JH, et al. Adventitial endothelial implants reduce matrix metalloproteinase-2 expression and increase luminal diameter in porcine arteriovenous grafts. J Vasc Surg. 2007;46(3):548–56.
Danoviz ME, Nakamuta JS, Marques FLN, Santos L, Alvarenga EC, Santos AA. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One. 2010;5, e12077.
Nakamuta JS, Danoviz ME, Marques FL, dos Santos L, Becker C, Gonçalves GA, et al. Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS One. 2009;4, e6005.
Danoviz ME, Bassaneze V, Nakamuta JS, dos Santos-Junior GR, Saint-Clair D, Bajgelman MC, et al. Adipose tissue-derived stem cells from humans and mice differ in proliferative capacity and genome stability in long-term cultures. Stem Cells Dev. 2011;20:661–70.
Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 2002;62:609–14. Gunasena KT, Villines PM, Critser ES, Critser JK: Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997; 12:101–106.
Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997;12:101–6.
Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7.
Nisolle M, Casanas-Roux F, Qu J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–9.
Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(4 Suppl):1550–8.
Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–5.
Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–22.
Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67(6):496–501.
Ansboro S, Greiser U, Barry F, Murphy M. Strategies for improved targeting of therapeutic cells: implications for tissue repair. Eur Cell Mater. 2012;23:310–8.
Sun Z, Tee BC, Kennedy KS, Kennedy PM, Kim DG, Mallery SR, et al. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: preliminary studies in a porcine model. PLoS One. 2013;8, e74672.
Jenkins HP, Janda R, Clarke J. Clinical and experimental observations on the use of gelatin sponge or foam. Surgery. 1946;20(1):124–32.
Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci: Mater Med. 2008;19(3):1173–82.
Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81.
Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85.
Gaytán M, Sánchez MA, Morales C, Bellido C, Millán Y, Martín de Las Mulas J, et al. Cyclic changes of the ovarian surface epithelium in the rat. Reproduction. 2005;129(3):311–21.
Luyckx V, Dolmans MM, Vanacker J, Scalercio SR, Donnez J, Amorim CA. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J Ovarian Res. 2013;6(1):83.
Luyckx V, Dolmans MM, Vanacker J, Legat C, Fortuño Moya C, Donnez J, et al. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril. 2014;101(4):1149–56.
Acknowledgments
We thank São Paulo Research Foundation (FAPESP, Pio XI St, 1500, Alto da Lapa, São Paulo/SP-Brazil, 05468-901) for grant support (process numbers: 2010/17897-5 and 2012/09469-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for post-doctoral scholarship; Esmeralda Miristene Eher, Angela Batista Santos, Maria Cristina Rodrigues Medeiros, and Sandra de Moraes Fernezlian (Immunohistochemistry Laboratory/Pathology Department/FMUSP) for technical assistance with immunohistochemistry assay; Leonard Medeiros da Silva (pathologist) for the help in the analysis of morphological data; and American Expert Journal for the english editing service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The Gelfoam scaffold could be a feasible and safe non-invasive technique for ASC delivery in the treatment of frozen-thawed ovarian autografts.
Rights and permissions
About this article
Cite this article
Damous, L.L., Nakamuta, J.S., Saturi de Carvalho, A.E.T. et al. Scaffold-based delivery of adipose tissue-derived stem cells in rat frozen-thawed ovarian autografts: preliminary studies in a rat model. J Assist Reprod Genet 32, 1285–1294 (2015). https://doi.org/10.1007/s10815-015-0527-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0527-x