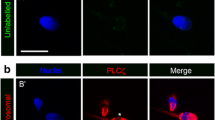
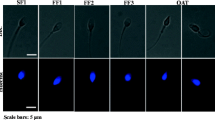
Abstract
Purpose
Intracytoplasmic sperm injection (ICSI) is widely used to achieve fertilization in the presence of severe male factor, resulting in high fertilization rates. Nevertheless, 1–3 % of couples experience complete fertilization failure after ICSI. When a male factor is identified, assisted oocyte activation (AOA) can help overcome fertilization failures. The objective of this study is to describe a case of repeated complete fertilization failures after ICSI with donor oocytes, and to investigate the molecular and functional aspects of phospholipase C zeta (PLCζ) protein in the patient semen.
Methods
The patient was a normozoospermic male who had previously fathered, through natural conception, four children by a different partner. Molecular and functional analysis of sperm-specific PLCζ in the patient and control samples by means of gene sequencing, immunocytochemistry, Western blot, mouse oocyte activation test (MOAT), and mouse oocyte calcium analysis (MOCA) were used.
Results
PLCζ expression levels and distribution were significantly disrupted, although MOAT and MOCA did not indicate a decrease in activation ability.
Conclusions
Normozoospermic males can have disrupted expression and distribution of PLCζ, and reduced activation ability after ICSI in human oocytes, despite their normal activation potential in functional testing using mouse oocytes. Discrepancy among molecular and functional data might exist, as mutations in the gene sequence may not be the only cause of alteration in PLCζ protein related to activation failures.

Similar content being viewed by others
References
Tesarik J, Sousa M. Mechanism of calcium oscillations in human oocytes: a two-store model. Mol Hum Reprod. 1996;2(6):383–6.
Mahutte NG, Arici A. Failed fertilization: is it predictable? Curr Opin Obstet Gynecol. 2003;15(3):211–8. doi:10.1097/01.gco.0000072858.73466.aa.
Sousa M, Tesarik J. Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Hum Reprod. 1994;9(12):2374–80.
Tesarik J, Mendoza C. In vitro fertilization by intracytoplasmic sperm injection. Bioessays. 1999;21(9):791–801. doi:10.1002/(SICI)1521-1878(199909)21:9<791::AID-BIES11>3.0.CO;2-Z.
Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Mol Hum Reprod. 2000;6(6):510–6.
Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94(2):520–6. doi:10.1016/j.fertnstert.2009.03.061.
Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28(5):560–71. doi:10.1016/j.rbmo.2014.01.008.
Eldar-Geva T, Brooks B, Margalioth EJ, Zylber-Haran E, Gal M, Silber SJ. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril. 2003;79 Suppl 3:1656–8.
Murase Y, Araki Y, Mizuno S, Kawaguchi C, Naito M, Yoshizawa M, et al. Pregnancy following chemical activation of oocytes in a couple with repeated failure of fertilization using ICSI: case report. Hum Reprod. 2004;19(7):1604–7. doi:10.1093/humrep/deh294.
Chi HJ, Koo JJ, Song SJ, Lee JY, Chang SS. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82(2):475–7. doi:10.1016/j.fertnstert.2004.01.038.
Chen J, Qian Y, Tan Y, Mima H. Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilisation failures during previous intracytoplasmic sperm injection treatment. Reprod Fertil Dev. 2010;22(5):852–5. doi:10.1071/RD09268.
Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod BioMed Online. 2008;17(5):662–8.
Vanden Meerschaut F, Nikiforaki D, De Roo C, Lierman S, Qian C, Schmitt-John T, et al. Comparison of pre- and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum Reprod. 2013;28(5):1190–8. doi:10.1093/humrep/det038.
WHO. WHO laboratory manual for the examination and processing of human semen. 5th Edition, 2010. Chapter 5.6. Preparing HIV-infected semen samples “Prepared samples should be tested by RT-PCR befor use, and only HIV-free samples used for ART”. 2010.
Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118(11):3671–81. doi:10.1172/JCI36942.
Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009;24(10):2417–28. doi:10.1093/humrep/dep207.
Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008;23(11):2513–22. doi:10.1093/humrep/den280.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5.
Vanden Meerschaut F, Leybaert L, Nikiforaki D, Qian C, Heindryckx B, De Sutter P. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod. 2013;28(1):87–98. doi:10.1093/humrep/des368.
Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, et al. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20(1):185–90. doi:10.1093/humrep/deh545.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCzeta): oocyte activation and clinical links to male factor infertility. Adv Biol Regul. 2013;53(3):292–308. doi:10.1016/j.jbior.2013.07.005.
Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, et al. Phospholipase Czeta rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril. 2013;99(1):76–85. doi:10.1016/j.fertnstert.2012.08.035.
Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, et al. Variance in total levels of phospholipase C zeta (PLC-zeta) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril. 2013;99(1):107–17. doi:10.1016/j.fertnstert.2012.09.001.
Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. Globozoospermia revisited. Hum Reprod Update. 2007;13(1):63–75. doi:10.1093/humupd/dml047.
Yanagida K. Complete fertilization failure in ICSI. Hum Cell. 2004;17(4):187–93.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2014;28(10):4434–40. doi:10.1096/fj.14-256495.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril. 2014;102(2):440–7. doi:10.1016/j.fertnstert.2014.05.003.
Rybouchkin A, Dozortsev D, de Sutter PD, Dhont M. Factors affecting the role of the spindle during early response of mouse oocytes to ethanol stimulation. J Exp Zool. 1996;275(6):469–75. doi:10.1002/(SICI)1097-010X(19960815)275:6<469::AID-JEZ9>3.0.CO;2-M.
Lee HC, Arny M, Grow D, Dumesic D, Fissore RA, Jellerette-Nolan T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet. 2014;31(6):749–56. doi:10.1007/s10815-014-0229-9.
Giannoulatou E, McVean G, Taylor IB, McGowan SJ, Maher GJ, Iqbal Z, et al. Contributions of intrinsic mutation rate and selfish selection to levels of de novo HRAS mutations in the paternal germline. Proc Natl Acad Sci U S A. 2013;110(50):20152–7. doi:10.1073/pnas.1311381110.
Maher GJ, Goriely A, Wilkie AO. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology. 2014;2(3):304–14. doi:10.1111/j.2047-2927.2013.00175.x.
Acknowledgments
The authors wish to thank Dr. Anna Ferrer, Dr. Elena Rebollo and Sara Casadesús for technical support.
Compliance with ethical standards
ᅟ
Informed consent
Informed consent was obtained from the couple involved in the study in order to analyze the semen samples and to report the case.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its subsequent amendments.
Financial support
Financial support for this study was provided in part by a fundamental clinical research mandate and a university grant.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded in part by Fundació Privada EUGIN, a fundamental clinical research mandate from the FWO-Vlaanderen to PDS, and a Ghent University grant to BH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Normozoospermic males can have disrupted expression and distribution of PLCζ, and reduced activation ability after ICSI in human oocytes, despite their normal activation potential in functional testing using mouse oocytes.
Mercè Durban and Montserrat Barragán contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Table 1
(DOCX 15 kb)
Suppl. Table 2
(DOCX 14 kb)
Suppl. Table 3
(DOCX 13 kb)
Suppl. Table 4
(DOCX 19 kb)
Suppl. Fig. 1
Representative patterns for the Ca2+ frequency scores (0, +, ++, +++). The frequency score is given by the number of calcium spikes recorded during 2 h post ICSI: a) +++ = >10; b) ++ = 3–10; c) + = 1–2; d) 0 = no spikes recorded (AU, arbitrary units). (GIF 15 kb)
Suppl. Fig. 2
Western Blot results from both blots. Immunoblotting was applied to determine protein expression of endogenous PLCζ (left panel), using tubulin as a loading control (right panel). The relative intensity of PLCζ bands (bar graph) were determined by densitometry referred to tubulin in fertile (white bar) and patient sperm (black bar). Different amounts of protein extracts, 50 (a) and 100 (b) μg, were applied and figures show the whole exposed blot. Arrows indicate bands for PLCζ (apparent MW of ≈70 kDa), * indicates unspecific bands. (GIF 158 kb)
Suppl. Fig. 3
Cellular localization of PLCζ protein in normozoospermic fertile human sperm. Representative image of PLCζ localization patterns by immunolocalization in fertile (a) and patient sperm (b). Squares represent corresponding upper (i) and lower (ii) panels in Fig. 1. Scale bars, 10 μm. (GIF 234 kb)
Rights and permissions
About this article
Cite this article
Durban, M., Barragán, M., Colodron, M. et al. PLCζ disruption with complete fertilization failure in normozoospermia. J Assist Reprod Genet 32, 879–886 (2015). https://doi.org/10.1007/s10815-015-0496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0496-0