Abstract
Purpose
To assess human fertilization and preimplantation embryo development in the presence and in the absence of carbon filtration
Methods
This is a retrospective cohort analysis of fresh, controlled ovarian hyperstimulation cycles as well as previously cryopreserved pronuclear stage embryo transfer cycles in a single IVF center. Embryo development and cycle-based outcomes were compared among three groups: 1) when carbon filtration was present, 2) when carbon filtration was absent, and 3) when carbon filtration had been restored.
Results
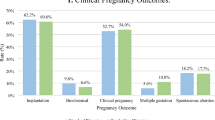
A total of 524 fresh cycles and 156 cryopreserved embryo cycles were analyzed. Fertilization, cleavage, and blastocyst conversion rates for fresh cycles all declined during the period of absent carbon filtration and recovered after the restoration of carbon filtration. Cryopreserved embryos that were thawed and cultured during the period of absent filtration did not have changes in cleavage or blastocyst conversion rates compared to periods where carbon filtration was present. Clinical pregnancy and live birth rates were unchanged among the three time periods.
Conclusions
The absence of carbon filtration in an IVF laboratory air handler is associated with poor fertilization and early embryo development for fresh cycles. Because development of previously frozen pronuclear stage embryos was unaffected, the lack of carbon filtration may preferentially affect embryos in the peri-fertilization period. Carbon filtration is an integral part to a successful human in-vitro fertilization laboratory.


Similar content being viewed by others
References
Ingebrigtsen R. Studies upon the characteristics of different culture media and their influence upon the growth of tissue outside of the organism. J Exp Med. 1912;16:421–31.
Whitten WK. Culture of tubal mouse ova. Nature. 1956;177:96.
Cohen J, Gilligan A, Esposito W, Schimmel T, Dale B. Ambient air and its potential effects on conception in vitro. Hum Reprod. 1997;12:1742–9.
Boone WR, Johnson JE, Locke AJ, Crane MMT, Price TM. Control of air quality in an assisted reproductive technology laboratory. Fertil Steril. 1999;71:150–4.
Ramalho de Carvalho BASA, de Oliveira I, Barreira Gomes Sobrinho D, Miura Nakagava H, Cesar Paes Barbosa A. Laboratory air quality upgrade from ISO class 7 to ISO class 5 improves ICSI outcomes in young infertile women. JBRA Assist Reprod. 2012;16:4.
Khoudja RY, Xu Y, Li T, Zhou C. Better IVF outcomes following improvements in laboratory air quality. J Assist Reprod Genet. 2013;30:69–76.
Souza Mdo C, Mancebo AC, da Rocha CA, Henriques CA, Souza MM, Cardoso FF. Evaluation of two incubation environments–ISO class 8 versus ISO class 5–on intracytoplasmic sperm injection cycle outcome. Fertil Steril. 2009;91:1780–4.
Merton JS, Vermeulen ZL, Otter T, Mullaart E, de Ruigh L, Hasler JF. Carbon-activated gas filtration during in vitro culture increased pregnancy rate following transfer of in vitro-produced bovine embryos. Theriogenology. 2007;67:1233–8.
Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2010;93:301–3.
Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. Effect of air quality on assisted human reproduction. Hum Reprod. 2010;25:1317–24.
Testart J, Lassalle B, Belaisch-Allart J, Hazout A, Forman R, Rainhorn JD, et al. High pregnancy rate after early human embryo freezing. Fertil Steril. 1986;46:268–72.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty : fertility & genetics beyond 1999. Carnforth: Parthenon Press; 1999. p. 378–88.
Hreinsson J. Quality management. In: Varghese A, Sjoblom P, Jayaprakasan K, editors. A practical guide to setting up an IVF Lab, embryo culture systems and running the unit. 1st ed. New Delhi: Jaypee Brothers Medical Pub; 2013. p. 87–93.
Mortimer D, Mortimer S. How are we doing? Benchmarking. In: Mortimer D, Mortimer S, editors. Quality and risk management in the IVF laboratory. 2nd ed. Cambridge: Cambridge University Press; 2015. p. 145–52.
Gianaroli L, Plachot M, van Kooij R, Al-Hasani S, Dawson K, DeVos A, et al. ESHRE guidelines for good practice in IVF laboratories. Committee of the Special Interest Group on Embryology of the European Society of Human Reproduction and Embryology. Hum Reprod. 2000;15:2241–6.
Van Voorhis BJ, Thomas M, Surrey ES, Sparks A. What do consistently high-performing in vitro fertilization programs in the U.S. do? Fertil Steril. 2010;94:1346–9.
Cohen J, Rieger D. Historical background of gamete and embryo culture. Embryo culture: methods and protocols, vol 1. New York: Springer; 2012.
Hall J, Gilligan A, Schimmel T, Cecchi M, Cohen J. The origin, effects and control of air pollution in laboratories used for human embryo culture. Hum Reprod. 1998;13 Suppl 4:146–55.
Acknowledgments
We would like to acknowledge Miriam Bridget Zimmerman, Clinical Professor of Biostatistics at the University of Iowa, for her assistance in the statistical analyses required for the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The absence of carbon filtration in an IVF laboratory air handler is associated with poor fertilization andearly embryo development for fresh cycles.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Cleavage Rates by Insemination Type, January–June. Cleavage rates are graphed for each January–June period from 4 years prior to, and 2 years after, the period of Absent carbon filtration. This change in cleavage rates had never been seen prior to, or since, the period of missing filtration and suggested variability not explained by minimal year-to-year changes expected in most IVF centers (XLSX 12.4 kb)
Rights and permissions
About this article
Cite this article
Munch, E.M., Sparks, A.E., Duran, H.E. et al. Lack of carbon air filtration impacts early embryo development. J Assist Reprod Genet 32, 1009–1017 (2015). https://doi.org/10.1007/s10815-015-0495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0495-1