Abstract
Purpose
This study evaluates the effect of low oxygen conditions (5 Vs 20 %) on buffalo embryo development. Expression patterns of key glucose metabolism genes (HK, PFK, LDH, PDH, G6PDH and Glut1) were assessed in buffalo oocytes and embryos cultured at 5 and 20 % oxygen and correlated with development rate.
Methods
Maturation rate was observed by determining MII stages by Aceto-orcein method and blastocyst formation was observed at 7 day post insemination (dpi). Expression levels of genes were determined by real time PCR in oocytes / embryos at 5 and 20 % O2.
Results
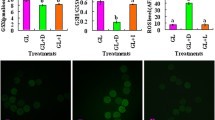
Oocyte maturation and blastocyst formation rates were significantly higher at 5 % O2 as compared to 20 % O2 (P < 0.05). The expression pattern of glycolytic genes (HK, PFK and G6PDH) indicated that oocytes and embryos under 5 % O2 tend to follow anaerobic glycolysis and pentose phosphate pathways to support optimum embryo development. Under 20 % O2, oocytes and embryos had high expression of PDH indicating higher oxidative phosphorylation. Further, less G6PDH expression at 20 % O2 was indicative of lower pentose phosphate activity. Higher expression of LDH was observed in oocytes and embryos under 20 % O2 indicating sub-optimal culture conditions. High Glut1 activity was observed in the oocytes / embryos at 5 % O2, indicative of high glucose uptake correlating with high expression of glycolytic genes.
Conclusion
The expression patterns of glucose metabolism genes could be a valuable indicator of the development potential of oocytes and embryos. The study indicates the importance of reduced oxygen conditions for production of good quality embryos.


Similar content being viewed by others
References
Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004;9:484–6.
Van Soom A, Yuan YQ, Peelman LJ, de Matos DG, Dewulf J, Laevens H, et al. Prevalence of apoptosis and inner cell allocation in bovine embryos cultured under different oxygen tensions with or without cysteine addition. Theriogenology. 2002;57:1453–65.
Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–97.
Du ZF, Wales RG. Glycolysis and glucose oxidation by the sheep conceptus at different oxygen concentrations. Reprod Fertil Dev. 1993;5:383–93.
Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol Reprod Dev. 2000;57:353–60.
Czyzyk-Krzeska MF. Molecular aspects of oxygen sensing in physiological adaptation to hypoxia. Respir Physiol. 1997;100:99–111.
Wengner RH, Gassman M. Oxygen and the hypoxia inducible factor-1. Biol Chem. 1997;378:609–16.
Ebert BL, Firth JF, Ratcliffe PJ. Hypoxia and mitochondria inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J Biol Chem. 1995;270:29083–9.
Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia inducible factor-1. J Biol Chem. 1994;269:23757–63.
Khurana NK, Wales RG. Effects of oxygen concentration on the metabolism of [U–14C]glucose by mouse morulae and early blastocysts in vitro. Reprod Fertil Dev. 1989;1:99–106.
Hooper K, Lane M, Gardner D. Reduced oxygen concentration increases mouse embryo development and oxidative metabolism. Theriogenology. 2001;55:334–40.
Sandt JJ, Schroeder AC, Eppig JJ. Culture media for mouse oocyte maturation affect subsequent embryonic development. Mol Reprod Dev. 1990;25:164–71.
Pinyopummintr T, Bavister BD. Optimum gas atmosphere for in vitro maturation and in vitro fertilization of bovine oocytes. Theriogenology. 1995;44:471–7.
Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86:1252–65.
Suresh KP, Nandi S, Mondal S. Factors affecting laboratory production of buffalo embryos: a meta-analysis. Theriogenology. 2009;72:978–85.
Kumar P, Verma A, Roy B, Rajput S, Ojha S, Anand S, et al. Effect of varying glucose concentrations during in vitro maturation and embryo culture on efficiency of in vitro embryo production in buffalo. Reprod Domest Anim. 2011;47:269–73.
Verma A, Kumar P, Rajput S, Roy B, De S, Datta TK. Embryonic genome activation events in buffalo (Bubalus bubalis) preimplantation embryos. Mol Reprod Dev. 2012;79:321–8.
Datta TK, Goswami SL. Time dynamics and chronology of meiotic progression of buffalo (Bubalus bubalis) oocytes during in vitro maturation. Buffalo J. 1999;1:53–60.
Kumar P, Yadav P, Verma A, Singh D, De S, Datta TK. Identification of stable reference genes for gene expression studies using quantitative real time PCR in buffalo oocytes and embryos. Reprod Domest Anim. 2012;47:e88–91.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–8.
Swain JE, Bormann CL, Clark SG, Walters EM, Wheeler MB, Krisher RL. Use of energy substrates by various stages preimplantation pig embryos produced in vivo and in vitro. Reproduction. 2002;123:253–60.
Preis KA, Seidel GE, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev. 2007;74:893–903.
Thompson JG. Comparison between in vivo-derived and in-vitro-produced pre-elongation embryos from domestic ruminants. Reprod Fertil Dev. 1997;9:341–54.
Khurana NK, Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biol Reprod. 2000;62:847–56.
Kim J, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–30.
Farrell PB, Foote RH. Beneficial effects of culturing rabbit zygotes to blastocyst in 5 % oxygen and 10 % carbon dioxide. J Reprod Fertil. 1995;103:127–30.
Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril. 2009;91:1459–61.
Iwata H, Akamatsu S, Minami N, Yamada M. Effects of antioxidants on the development of bovine IVM/IVF embryos in various concentrations of glucose. Theriogenology. 1998;50:365–75.
Marques MG, de Barros FR, Goissis MD, Cavalcanti PV, Viana CH, Assumpcao ME, et al. Effect of low oxygen tension atmosphere and maturation media supplementation on nuclear maturation, cortical granules migration and sperm penetration in swine in vitro fertilization. Reprod Domest Anim. 2012;47:491–507.
Voelkel SA, Hu YX. Effect of gas atmosphere on the development of one-cell bovine embryos in two culture systems. Theriogenology. 1992;37:1117–31.
Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118:47–55.
Machaty Z, Thompson JG, Abeydeera LR, Day BN, Prather RS. Inhibitors of mitochondrial ATP production at the time of compaction improve development of in vitro produced porcine embryos. Mol Reprod Dev. 2001;58:39–44.
Hashimoto S, Minami N, Yamada M, Imai H. An excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev. 2000;56:520–6.
Leese HJ, Baumann CG, Brison D, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–72.
Sturmey RG, Hawkhead J, Barker EA, Leese HJ. DNA damage and metabolic activity in the preimplantation embryo. Hum Reprod. 2009;24:81–91.
Kroener L, Ambartsumyan G, Jones CB, Dumesic D, Surrey M, Munne S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98:876–80.
Scott Jr RT, Upham K, Forman E, Hong K, Scott K, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilitzation implantation and delivery rates; a randomized controlled trial. Fertil Steril. 2013;100:697–703.
Brad AM, Bormann CL, Swain JE, Durkin RE, Johnson AE, Clifford AL, et al. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol Reprod Dev. 2003;64:492–8.
Herrick JR, Brad AM, Krisher RL, Pope WF. Intracellular adenosine triphosphate and glutathione concentrations in oocytes from first estrous, multi-estrous, and testosterone-treated gilts. Anim Reprod Sci. 2003;78:123–31.
Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/ blastocyst formation of bovine embryos. Theriogenology. 2000;54:741–56.
Kind KL, Collett RA, Harvey AJ, Thompson JG. Oxygen-regulated expression of GLUT-1, GLUT-3, and VEGF in the mouse blastocyst. Mol Reprod Dev. 2005;70:37–44.
Bermejo-Álvarez P, Lonergan P, Rizos D, Gutiérrez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod BioMed Online. 2010;20:341–9.
Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–19.
Morita Y, Osamu T, Iwao H. Expression and possible function of glucose transporter protein GLUT-1 during preimplantation mouse development from oocytes to blastocysts. Biochem Biophys Res Commun. 1992;188:8–15.
Hogan A, Heyner S, Charron MJ, Copeland NG, Gilbert DJ, Jekins NA, et al. Glucose transporter gene expression in early mouse embryos. Development. 1991;113:363–72.
Pantaleon M, Harvey MB, Pascoe WS. Glucose transporter GLUT3: ontogeny, targeting, and role in mouse blastocyst. Proc Natl Acad Sci U S A. 1997;94:3795–800.
Carayannopoulos MO, Chi MM, Cui Y. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–8.
Acknowledgments
Critical inputs of Dr.R.K. Sharma, PS, Biochemistry Div, NDRI for interpreting the data, of Mr. Gian Singh, Technical Officer, NDRI computer section for statistical analysis and fund received from NAIP C-1056 and NAE projects of ICAR to the corresponding author are thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The expression pattern of glucose metabolism genes was found to be correlated with improved development of buffalo oocytes / embryos under low oxygen condition.
Rights and permissions
About this article
Cite this article
Kumar, P., Verma, A., Kumar, M. et al. Expression pattern of glucose metabolism genes correlate with development rate of buffalo oocytes and embryos in vitro under low oxygen condition. J Assist Reprod Genet 32, 471–478 (2015). https://doi.org/10.1007/s10815-014-0418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0418-6