Abstract
Purpose
To investigate the expression of GRIM-19 (Gene associated with retinoid-interferon-induced mortality 19) in mouse oocytes and preimplantation embryos, and to study the effect of GRIM-19 on the developmental competence of mouse oocytes and embryos.
Methods
GRIM-19 was evaluated at both mRNA and protein levels. The expression of GRIM-19 gene was downregulated in mouse oocytes cultured in vitro by specific small interfering RNA (siRNA) injection, while the activity of GRIM-19 was decreased by microinjection of a GRIM-19 antibody into the cytoplasm of germinal vesicle (GV) oocytes. Oocytes matured in vitro were then fertilized by intracytoplasmic sperm injection (ICSI), followed by observation and evaluation of fertilization rate, cleavage rate, blastocyst formation rate and implantation rate.
Results
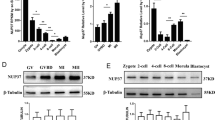
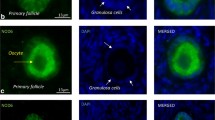
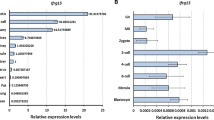
GRIM-19 is expressed throughout oocyte maturation and preimplantation embryo development stages. GRIM-19 was localized primarily in the cytoplasm of all cells examined. Downregulation of gene expression and activity of GRIM-19 resulted in decreased oocyte viability, potency of oocyte maturation, embryo development and implantation.
Conclusions
GRIM-19 may play important roles in mouse oogenesis and early embryonic development and implantation.




Similar content being viewed by others
References
Duranthon V, Renard JP. The developmental competence of mammalian oocytes: a convenient but biologically fuzzy concept. Theriogenology. 2001;55(6):1277–89.
Sirard MA. Resumption of meiosis: mechanism involved in meiotic progression and its relation with developmental competence. Theriogenology. 2001;55(6):1241–54.
Krey LC, Grifo JA. Poor embryo quality: the answer lies (mostly) in the egg. Fertil Steril. 2001;75(3):466–8.
Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion. 2004;4(5–6):577–600.
Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112(4):481–90.
Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283(5407):1488–93.
Coenen MJ, van den Heuvel LP, Smeitink JA. Mitochondrial oxidative phosphorylation system assembly in man: recent achievements. Curr Opin Neurol. 2001;14(6):777–81.
McConnell JM, Petrie L. Mitochondrial DNA turnover occurs during preimplantation development and can be modulated by environmental factors. Reprod Biomed Online. 2004;9(4):418–24.
Cohen J, Scott R, Alikani M, Schimmel T, Munne S, Levron J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4(3):269–80.
Dale B, Wilding M, Botta G, Rasile M, Marino M, Di Matteo L, et al. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16(7):1469–72.
Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16(3):513–6.
El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131(2):233–45.
Angell JE, Lindner DJ, Shapiro PS, Hofmann ER, Kalvakolanu DV. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J Biol Chem. 2000;275(43):33416–26.
Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT, et al. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J. 2003;22(6):1325–35.
Zhang J, Yang J, Roy SK, Tininini S, Hu J, Bromberg JF, et al. The cell death regulator GRIM-19 is an inhibitor of signal transducer and activator of transcription 3. Proc Natl Acad Sci U S A. 2003;100(16):9342–7.
Maximo V, Botelho T, Capela J, Soares P, Lima J, Taveira A, et al. Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br J Cancer. 2005;92(10):1892–8.
Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH: ubiquinone oxidoreductase (complex I). J Biol Chem. 2001;276(42):38345–8.
Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, et al. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol. 2004;24(19):8447–56.
Huang G, Chen Y, Lu H, Cao X. Coupling mitochondrial respiratory chain to cell death: an essential role of mitochondrial complex I in the interferon-beta and retinoic acid-induced cancer cell death. Cell Death Differ. 2007;14(2):327–37.
Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev Biol. 2007;7:14.
Jeong YJ, Choi HW, Shin HS, Cui XS, Kim NH, Gerton GL, et al. Optimization of real time RT-PCR methods for the analysis of gene expression in mouse eggs and preimplantation embryos. Mol Reprod Dev. 2005;71(3):284–9.
Dai Y, Lee C, Hutchings A, Sun Y, Moor R. Selective requirement for Cdc25C protein synthesis during meiotic progression in porcine oocytes. Biol Reprod. 2000;62(3):519–32.
Stewart BM, Block J, Morelli P, Navarette AE, Amstalden M, Bonilla L et al. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J Dairy Sci 94(7):3437–45
Yamagata K, Suetsugu R, Wakayama T. Assessment of chromosomal integrity using a novel live-cell imaging technique in mouse embryos produced by intracytoplasmic sperm injection. Hum Reprod. 2009;24(10):2490–9.
Yamagata K, Suetsugu R, Wakayama T. Long-term, six-dimensional live-cell imaging for the mouse preimplantation embryo that does not affect full-term development. J Reprod Dev. 2009;55(3):343–50.
Ohta H, Sakaide Y, Wakayama T. Long-term preservation of mouse spermatozoa as frozen testicular sections. J Reprod Dev. 2008;54(4):295–8.
Hiendleder S, Wolf E. The mitochondrial genome in embryo technologies. Reprod Domest Anim. 2003;38(4):290–304.
Kondo I, Suganuma N, Ando T, Asada Y, Furuhashi M, Tomoda Y. Clinical factors for successful cryopreserved-thawed embryo transfer. J Assist Reprod Genet. 1996;13(3):201–6.
Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64(3):904–9.
Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J Biol Chem. 2006;281(43):32724–7.
Murray J, Zhang B, Taylor SW, Oglesbee D, Fahy E, Marusich MF, et al. The subunit composition of the human NADH dehydrogenase obtained by rapid one-step immunopurification. J Biol Chem. 2003;278(16):13619–22.
Sakkas D, Shoukir Y, Chardonnens D, Bianchi PG, Campana A. Early cleavage of human embryos to the two-cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1998;13(1):182–7.
Van Royen E, Mangelschots K, De Neubourg D, Valkenburg M, Van de Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14(9):2345–9.
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15(11):2394–403.
Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8(1):45–58.
Lee SH, Han JH, Cho SW, Cha KE, Park SE, Cha KY. Mitochondrial ATPase 6 gene expression in unfertilized oocytes and cleavage-stage embryos. Fertil Steril. 2000;73(5):1001–5.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81370711), National Natural Science Foundation of China (grant number 30901603), Doctor Foundation of Shandong Province (grant number BS2009SW031), and Shandong Family Planning Commission Foundation (grant number 2009–8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chao, L., Wang, X., Yang, Y. et al. Downregulation of gene expression and activity of GRIM-19 affects mouse oocyte viability, maturation, embryo development and implantation. J Assist Reprod Genet 32, 461–470 (2015). https://doi.org/10.1007/s10815-014-0413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0413-y