Abstract
Purpose
Since many transferred, good morphology embryos fail to implant, technologies to identify embryos with high developmental potential would be beneficial. The Eeva™ (Early Embryo Viability Assessment) Test, a prognostic test based on automated detection and analysis of time-lapse imaging information, has been shown to benefit embryo selection specificity for a panel of three highly experienced embryologists (Conaghan et al., 2013). Here we examined if adjunctive use of Eeva Test results following morphological assessment would allow embryologists with diverse clinical backgrounds to consistently improve the selection of embryos with high developmental potential.
Methods
Prospective, double-blinded multi-center study with 54 patients undergoing blastocyst transfer cycles consented to have embryos imaged using the Eeva System, which automatically measures key cell division timings and categorizes embryos into groups based on developmental potential. Five embryologists of diverse clinical practices, laboratory training, and geographical areas predicted blastocyst formation using day 3 morphology alone and day 3 morphology followed by Eeva Test results. Odds ratio (OR) and diagnostic performance measures were calculated by comparing prediction results to true blastocyst outcomes.
Results
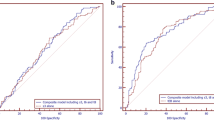
When Eeva Test results were used adjunctively to traditional morphology to help predict blastocyst formation among embryos graded good or fair on day 3, the OR was 2.57 (95 % CI=1.88–3.51). The OR using morphology alone was 1.68 (95 % CI=1.29–2.19). Adjunct use of the Eeva Test reduced the variability in prediction performance across all five embryologists: the variability was reduced from a range of 1.06 (OR=1.14 to 2.20) to a range of 0.45 (OR=2.33 to 2.78).
Conclusions
The Eeva Test, an automated, time-lapse enabled prognostic test, used adjunctively with morphology, is informative in helping embryologists with various levels of experience select embryos with high developmental potential.




Similar content being viewed by others
References
Van Montfoort AP, Dumoulin JC, Kester AD, Evers JL. Early cleavage is a valuable addition to existing embryo selection parameters: a study using single embryo transfers. Hum Reprod. 2004;19(9):2103–8. doi:10.1093/humrep/deh385.
Diamond MP, Willman S, Chenette P, Cedars MI. The clinical need for a method of identification of embryos destined to become a blastocyst in assisted reproductive technology cycles. J Assist Reprod Genet. 2012;29(5):391–6. doi:10.1007/s10815-012-9732-z.
Practice Committee of Society for Assisted Reproductive T, Practice Committee of American Society for Reproductive M. Elective single-embryo transfer. Fertil Steril. 2012;97(4):835–42. doi:10.1016/j.fertnstert.2011.11.050.
Paternot G, Devroe J, Debrock S, D’Hooghe TM, Spiessens C. Intra- and inter-observer analysis in the morphological assessment of early-stage embryos. Reprod Biol Endocrinol: RB&E. 2009;7:105. doi:10.1186/1477-7827-7-105.
Paternot G, Debrock S, D’Hooghe T, Spiessens C. Computer-assisted embryo selection: a benefit in the evaluation of embryo quality? Reprod Biomed Online. 2011;23(3):347–54. doi:10.1016/j.rbmo.2011.05.007.
Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28(7):569–73. doi:10.1007/s10815-011-9549-1.
Gleicher N, Barad DH. A review of, and commentary on, the ongoing second clinical introduction of preimplantation genetic screening (PGS) to routine IVF practice. J Assist Reprod Genet. 2012;29(11):1159–66. doi:10.1007/s10815-012-9871-2.
Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004;74(4):599–609. doi:10.1086/382897.
Horsthemke B, Ludwig M. Assisted reproduction: the epigenetic perspective. Hum Reprod Update. 2005;11(5):473–82. doi:10.1093/humupd/dmi022.
Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91(2):305–15. doi:10.1016/j.fertnstert.2009.01.002.
Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99(3):731–7. doi:10.1016/j.fertnstert.2012.11.023.
Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive T. Blastocyst culture and transfer in clinical-assisted reproduction: a committee opinion. Fertility and sterility. 2013;99(3):667–72. doi:10.1016/j.fertnstert.2013.01.087.
Clua E, Tur R, Coroleu B, Boada M, Rodriguez I, Barri PN, et al. Elective single-embryo transfer in oocyte donation programmes: should it be the rule? Reprod Biomed Online. 2012;25(6):642–8. doi:10.1016/j.rbmo.2012.09.008.
Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100(2):412–9 e5. doi:10.1016/j.fertnstert.2013.04.021.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24. doi:10.1186/1755-8166-5-24.
Forman EJ, Upham KM, Cheng M, Zhao T, Hong KH, Treff NR, et al. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 2013;100(3):718–24. doi:10.1016/j.fertnstert.2013.04.043.
Chen AA, Shen S. Predicting Embryo Developmental Potential and Viability Using Automated Time-Lapse Analysis (Eeva Test). 2013:377–89. doi:10.1007/978-1-4614-8376-2_22.
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99(4):1035–43. doi:10.1016/j.fertnstert.2013.01.143.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–21. doi:10.1038/nbt.1686.
Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98(6):1481–9 e10. doi:10.1016/j.fertnstert.2012.08.016.
Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–71. doi:10.1093/humrep/der256.
Dal Canto M, Coticchio G, Mignini Renzini M, De Ponti E, Novara PV, Brambillasca F, et al. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online. 2012;25(5):474–80. doi:10.1016/j.rbmo.2012.07.016.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98(6):1458–63. doi:10.1016/j.fertnstert.2012.07.1135.
Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun. 2012;3:1251. doi:10.1038/ncomms2249.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013;26(5):477–85. doi:10.1016/j.rbmo.2013.02.006.
Basile N, Nogales Mdel C, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101(3):699–704. doi:10.1016/j.fertnstert.2013.12.005.
Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into society for assisted reproductive technology clinic outcomes reporting system: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95(6):1985–9. doi:10.1016/j.fertnstert.2011.02.009.
Rubin DB. Multiple Imputation for Nonresponse in Surveys. 1987.
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10:CD001431. doi:10.1002/14651858.CD001431.pub3.
Baxter Bendus AE, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril. 2006;86(6):1608–15. doi:10.1016/j.fertnstert.2006.05.037.
Harper JC, Harton G. The use of arrays in preimplantation genetic diagnosis and screening. Fertil Steril. 2010;94(4):1173–7. doi:10.1016/j.fertnstert.2010.04.064.
Palmer SS, Barnhart KT. Biomarkers in reproductive medicine: the promise, and can it be fulfilled? Fertil Steril. 2013;99(4):954–62. doi:10.1016/j.fertnstert.2012.11.019.
Harper J, Cristina Magli M, Lundin K, Barratt CLR, Brison D. When and how should new technology be introduced into the IVF laboratory? Hum Reprod. 2012;27(2):303–13. doi:10.1093/humrep/der414.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod Biomed Online. 2013;27(2):140–6. doi:10.1016/j.rbmo.2013.04.013.
Acknowledgments
We acknowledge Auxogyn, Inc. for sponsoring this study, the clinical, scientific and algorithm groups at Auxogyn for insightful discussion, and the physicians, embryologists and patients who participated in the development and validation of the Eeva Test.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The Eeva Test is highly informative and, when used adjunctively to morphology, allows embryologists from diverse clinical backgrounds to consistently improve embryo selection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Morphological grade assignments (A, B, C, and D) made by five embryologists using day 3 morphology data for n=758 embryos. (GIF 52 kb)
Rights and permissions
About this article
Cite this article
Diamond, M.P., Suraj, V., Behnke, E.J. et al. Using the Eeva Test™ adjunctively to traditional day 3 morphology is informative for consistent embryo assessment within a panel of embryologists with diverse experience. J Assist Reprod Genet 32, 61–68 (2015). https://doi.org/10.1007/s10815-014-0366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0366-1