Abstract
Purpose
Embryonic poly(A)-binding protein (EPAB) and poly(A)-binding protein, cytoplasmic 1 (PABPC1) bind poly(A) tails of mRNAs and mediate their translational regulation in germ cells and early preimplantation embryos. Although expression patterns and possible functions of the Epab and Pabpc1 genes have been examined in vertebrate germ cells and early embryos, their expression levels and cellular localizations in the postnatal mouse ovaries remained elusive.
Methods
In the present study, we first aimed to characterize expression levels of the Epab and Pabpc1 genes in the prepubertal (1-, 2-, and 3-week old), pubertal (4-, 5-, and 6-week old), postpubertal (16-week and 18-week old), and aged (52-, 60-, and 72-week old) mouse ovaries by using quantitative real-time polymerase chain reaction (qRT-PCR).
Results
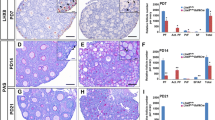
Epab mRNA was predominantly expressed in the prepubertal ovaries when compared to later developmental periods. However, Pabpc1 transcript was highly generated in the prepubertal and pubertal mouse ovaries except for 1-week old ovary than those of other developmental terms. In the prepubertal mouse ovaries, RNA in situ hybridization localized both Epab and Pabpc1 transcripts in the cytoplasm of oocytes and granulosa cells of all follicular stages. Consistently, Epab and Pabpc1 gene expression were detected in the cumulus cells and MII oocytes obtained from cumulus oocyte complexes (COCs). Ovarian follicle counting in the postnatal ovaries revealed that total number of follicles was higher in the prepubertal ovaries in comparison with later stages of development.
Conclusion
As a result, Epab and Pabpc1 expression exhibit differences at postnatal ovary development stages and both genes are transcribed in the granulosa cells and oocytes. These findings suggest that EPAB may predominantly play roles in translational regulation of the mRNAs during early oogenesis and folliculogenesis, but PABPC1 most likely perform these roles in the later terms of ovarian development along with EPAB protein.





Similar content being viewed by others
References
Wilkie GS, Gautier P, Lawson D, Gray NK. Embryonic poly(A)-binding protein stimulates translation in germ cells. Mol Cell Biol. 2005;25:2060–71.
Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–88.
Kim JH, Richter JD. RINGO/cdk1 and CPEB mediate poly(A) tail stabilization and translational regulation by ePAB. Genes Dev. 2007;21:2571–9.
Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci U S A. 2005;102:367–72.
Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB). Mol Hum Reprod. 2008;14:581–8.
Ozturk S, Guzeloglu-Kayisli O, Demir N, Sozen B, Ilbay O, Lalioti MD, et al. Epab and Pabpc1 are differentially expressed during male germ cell development. Reprod Sci. 2012;19:911–22.
Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, et al. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 2012;446:47–58.
Ben-Or S. Morphological and functional development of the ovary of the mouse. I. Morphology and histochemistry of the developing ovary in normal conditions and after FSH treatment. J Embryol Exp Morpholog. 1963;11:1–11.
Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh). 1969;62:98–116.
Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–54.
Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903–10.
Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312.
Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–40.
Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–49.
Bachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol. 2005;68:49–84.
Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod BioMed Online. 2007;14:758–64.
Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci. 2006;63:579–90.
Bachvarova R. Gene expression during oogenesis and oocyte development in mammals.Dev Biol (N Y 1985). 1985;1:453–524.
Song JL, Wessel GM. How to make an egg: transcriptional regulation in oocytes. Differentiation. 2005;73:1–17.
Danilovich N, Ram SM. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41:117–22.
Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93.
Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–50.
Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–32.
Brook M, Smith JW, Gray NK. The DAZL and PABP families: RNA-binding proteins with interrelated roles in translational control in oocytes. Reproduction. 2009;137:595–617.
Guzeloglu-Kayisli O, Lalioti MD, Babayev E, Torrealday S, Karakaya C Seli E. Human embryonic poly(A)-binding protein (EPAB) alternative splicing is differentially regulated in human oocytes and embryos. Mol Hum Reprod. 2013
Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 2006;20:199–209.
Friend K, Brook M, Bezirci FB, Sheets MD, Gray NK, Seli E. Embryonic poly(A)-binding protein (ePAB) phosphorylation is required for Xenopus oocyte maturation. Biochem J. 2012;445:93–100.
Acknowledgments
This study was supported by TUBITAK Fund (Grant No. 111S333). The authors thank to Onur Bakiner, PhD for corrections to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Understanding importance of the PABP proteins during postnatal ovarian development would provide us to create new treatments for various types of ovarian diseases.
Rights and permissions
About this article
Cite this article
Ozturk, S., Sozen, B. & Demir, N. Epab and Pabpc1 are differentially expressed in the postnatal mouse ovaries. J Assist Reprod Genet 32, 137–146 (2015). https://doi.org/10.1007/s10815-014-0362-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0362-5