Abstract
Purpose
To compare the efficacy of swim-up and DGC in improving sperm deformity and DNA fragmentation and to determine which method is better in teratozoospermic patients requiring artificial reproduction.
Methods
The present study compared the effects of swim-up and density gradient centrifugation (DGC), the two most commonly used semen preparation methods, on sperm deformity rate and DNA fragmentation index (DFI) in semen samples from teratozoospermic patients.
Results
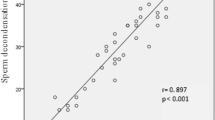
The results demonstrated that both swim-up and DGC yielded a significantly lower sperm deformity rate and DFI in comparison to unprocessed whole semen, with DGC having more favorable results. Sperm deformity rate in unprocessed whole semen samples was significantly lower in the 20–29 age group than in the 40-49 age group, but no significant difference was observed in DFI between different age groups. There was no significant correlation between sperm deformity rate and DFI.
Conclusions
Our findings suggest that enrichment of sperm with normal morphology and intact DNA in teratozoospermic patients could be achieved by both DGC and swim-up procedures, and that DGC is a better method.


Similar content being viewed by others
References
Burrello N, Arcidiacono G, Vicari E, Asero P, Di Benedetto D, et al. Morphologically normal spermatozoa of patients with secretory oligo-astheno-teratozoospermia have an increased aneuploidy rate. Hum Reprod. 2004;19:2298–302.
Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, et al. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–15.
Henkel R. Sperm preparation: state-of-the-art–physiological aspects and application of advanced sperm preparation methods. Asian J Androl. 2012;14:260–9.
Brahem S, Mehdi M, Elghezal H, Saad A. Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet. 2011;28:41–8.
Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2004;3:CD004507.
Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007;4:CD004507.
Henkel RR, Schill WB. Sperm preparation for art. Reprod Biol Endocrinol. 2003;1:108.
van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59:86–91.
Avendaño C, Oehninger S. DNA fragmentation in morphologically normal spermatozoa: how much should we be concerned in the ICSI era? J Androl. 2011;32:356–63.
Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85.
Simon L, Lewis SE. Sperm DNA damage or progressive motility: which one is the better predictor of fertilization in vitro? Syst Biol Reprod Med. 2011;57:133–8.
World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. New York: Cambridge University Press; 2010.
Paasch U, Grunewald S, Glander HJ. Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl. 2007;65:515–25.
Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557–63.
Hammadeh ME, Kühnen A, Amer AS, Rosenbaum P, Schmidt W. Comparison of sperm preparation methods: effect on chromatin and morphology recovery rates and their consequences on the clinical outcome after in vitro fertilization embryo transfer. Int J Androl. 2001;24:360–8.
Ng FL, Liu DY, Baker HW. Comparison of Percoll, mini-Percoll and swim-up methods of sperm preparation from abnormal semen samples. Hum Reprod. 1992;7:261–6.
Chiamchanya C, Kaewnoonual N, Visutakul P, Manochantr S, Chaiya J. Comparative study of the effects of three semen preparation media on semen analysis, DNA damage and protamine deficiency, and the correlation between DNA integrity and sperm parameters. Asian J Androl. 2010;12:271–7.
Borges Jr E, Setti AS, Vingris L, Figueira Rde C, Braga DP, et al. Intracytoplasmic morphologically selected sperm injection outcomes: the role of sperm preparation techniques. J Assist Reprod Genet. 2013;30:849–54.
Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, et al. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 2000;15:1112–6.
Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56:1081–4.
Monqaut AL, Zavaleta C, Lopez G, Lafuente R, Brassesco M. Use of high-magnification microscopy for the assessment of sperm recovered after two different sperm processing methods. Fertil Steril. 2011;95:277–80.
Ahmad L, Jalali S, Shami SA, Akram Z. Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Arch Androl. 2007;53:325–38.
Hashimoto S, Goda S, Akamatsu Y, Yamanaka M, Morimoto Y. Effects of sperm preparation on sperm DNA fragmentation and morphology. RBM Online. 2008;16:S28.
Ricci G, Perticarari S, Boscolo R. MonticoM, Guaschino S et al. Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrifugation technique. Fertil Steril. 2009;91:632–8.
Siddighi S, Chan CA, Patton WC, Jacobson JD, Chan PJ. Male age and sperm necrosis in assisted reproductive technologies. Urol Int. 2007;79:231–4.
Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–43.
Dakouane M, Albert M, Bergere M, Sabbagh C, Brayotel F, et al. Aging and spermatogenesis: an histologic, cytogenetic and apoptosis study. Gynecol Obstet Fertil. 2005;33:659–64.
Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, et al. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009;91:1801–5.
Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–30.
Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–6.
Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, et al. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100.
Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, et al. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–15.
Matsuura R, Takeuchi T, Yoshida A. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl. 2010;12:753–9.
Acknowledgments
This work was supported by the Social Development Research Project for Science and Technology of Shaanxi Province, China (No. 2011 K15-02-01), the Research Project of Health Department of Shaanxi Province, China (No. 2012D6), and Science and Technology Planning Project of Guangdong Province, China (No. 2012B040304010)
Author information
Authors and Affiliations
Corresponding author
Additional information
Xia Xue and Wan-Shan Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xue, X., Wang, WS., Shi, JZ. et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 31, 1161–1166 (2014). https://doi.org/10.1007/s10815-014-0287-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0287-z