Abstract
Purpose
To develop an efficient protocol for isolation, purification and long-term culture of spermatogonial stem cell (SSC) in goat.
Methods
The isolation of SSC was performed by testicular disaggregation by enzymatic digestion using collagenase IV, trypsin and DNase I. Further SSCs were enriched using Percoll density gradient centrifugation. The purity of SSCs was assessed by immunocytochemistry (ICC) using α6 integrin. The SSCs were co-cultured on Sertoli cell feeder layer. The SSC colonies were characterized by studying the expression of SSC specific markers (viz., α6 integrin and PLZF) using ICC. The abundance of mRNAs encoding the markers of SSC (viz., β1 integrin and Oct-4) and Sertoli cells (viz., vimentin) was also assayed using quantitative real-time PCR (qPCR).
Results
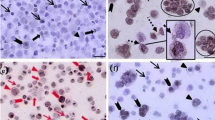
The viability of isolated testicular cells was > 90 % and the Percoll density gradient method resulted in 3.65 folds enrichment with a purity of 82.5 %. Co-culturing of SSCs with Sertoli cell feeder layer allowed the maintenance of stable SSC colonies even after one and half months of culture. The results of ICC analysis showed the expression of α6 integrin and PLZF in almost all the SSC colonies. qPCR analysis revealed a differential expression of mRNAs encoding β1 integrin, Oct-4 and vimentin markers.
Conclusion
Results of this study demonstrate a simple enzymatic digestion and Percoll density gradient method for isolation and enrichment of SSCs, and suitability of Sertoli cell feeder layer for long term in vitro culture of SSC in goats. Results also suggest the possible application of non-caprine antibodies against SSC specific markers for the identification and subsequent assessment of SSCs in goats.




Similar content being viewed by others
References
Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–78.
Caires K, Broady J, Mclean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol. 2010;205:133–45.
Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod. 2013;28:897–907.
de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98.
Kanatsu-Shinohara M, Kato M, Takehashi M, Morimoto H, Takashima S, Chuma S, et al. Production of transgenic rats via lentiviral transduction and xenogeneic transplantation of spermatogonial stem cells. Biol Reprod. 2008;79:1121–8.
Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS ONE. 2013;8:e77715.
Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, et al. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2002;99:14931–6.
Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–7.
He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–72.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Hosseini SE, Vahdati A, et al. Short-term in-vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J Assist Reprod Genet. 2012;29:39–46.
Nasiri Z, Hosseini SM, Hajian M, Abedi P, Bahadorani M, Baharvand H, et al. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells in-vitro. Theriogenology. 2012;77:1519–28.
Goharbakhsh L, Mohazzab A, Salehkhou S, Heidari M, Zarnani AH, Parivar K, et al. Isolation and culture of human spermatogonial stem cells derived from testis biopsy. Avicenna J Med Biotech. 2013;5:54–61.
Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9 (141).
Mahla RS, Reddy N, Goel S. Spermatogonial stem cells (SSCs) in buffalo (Bubalus bubalis) testis. PLoS One. 2012;7e36020.
van Pelt AMM, Morena AR, van Dissel-Emiliani FMF, Boitani C, Gaemers IC, de Rooij DG, et al. Isolation of the synchronized a spermatogonia from adult vitamin a-deficient rat testes. Biol Reprod. 1996;55:439–44.
Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124:85–94.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci. 2000;97:8346–51.
Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Orwig PC, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–6.
Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274:158–70.
Bruno AH, Giassetti MI, de Barros FRO, Siqueira AF, Mendes CM, Lopes E, et al. Expression of molecular markers for bovine spermatogonial stem cells in prepubertal and adults Nelore. Anim Reprod. 2013;10:602.
Piravar Z, Jeddi-Tehrani M, Sadeghi MR, Mohazzab A, Eidi A, Akhondi MM. In vitro culture of human testicular stem cells on feeder-free condition. J Reprod Infertil. 2013;14:17–22.
Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84:97–105.
Rastegar T, Minaee MB, Roudkenar MH, Kashani IR, Amidi F, Abolhasani F, et al. Improvement of expression of α6 and β1 integrins by the Co-culture of adult mouse spermatogonial stem cells with SIM mouse embryonic fibroblast cells (STO) and growth factors. Iran J Basic Med Sci. 2013;16:134–9.
Oatley J, de Avila DM, McLean DJ, Griswold MD, Reeves JJ. Transplantation of bovine germinal cells into mouse testes. J Anim Sci. 2002;80:1925–31.
Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod. 2004;71:494–501.
Aponte PM, Soda T, van de Kant HJ, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2006;65:1828–47.
Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cell Dev-An. 2009;45:281–9.
Olive V, Cuzin F. The spermatogonial stem cell: from basic knowledge to transgenic technology. Int J Biochem Cell Biol. 2005;37:246–50.
Zhao Y, Yu M, Wang L, Li Y, Fan J, Yang Q, et al. Spontaneous uptake of exogenous DNA by goat spermatozoa and selection of donor bucks for sperm-mediated gene transfer. Mol Biol Rep. 2012;39:2659–64.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6.
Hamra FK, Chapman KM, Nguyen DM, Williams AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–5.
Hamra FK, Chapman KM, Wu Z, Garbers DL. Isolating highly pure rat spermatogonial stem cells in culture. Methods Mol Biol. 2008;450:163–79.
Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–57.
Kala S, Kaushik R, Singh KP, Kadam PH, Singh MK, Manik RS, et al. In vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. J Assist Reprod Genet. 2012;29:1335–42.
Kadam PH, Kala S, Agrawal H, Singh KP, Singh MK, Chauhan MS, et al. Effects of glial cell line-derived neurotrophic factor, fibroblast growth factor 2 and epidermal growth factor on proliferation and the expression of some genes in buffalo (Bubalus bubalis) spermatogonial cells. Reprod Fert Develop. 2013;25:1149–57.
Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–306.
Kaul G, Kumar S, Kumari S. Enrichment of CD9+ spermatogonial stem cells from goat (Capra aegagrus hircus) testis using magnetic microbeads. Stem Cell Discovery. 2012;2:92–9.
Pramod RK, Sharma SK, Singhi A, Pan S, Mitra A. Differential ovarian morphometry and follicular expression of BMP15, GDF9 and BMPR1B influence the prolificacy in goat. Reprod Domest Anim. 2013;48:803–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCTCt method. Methods. 2001;25:402–8.
Han SY, Gupta MK, Uhm SJ, Lee HT. Isolation and in vitro culture of Pig spermatogonial stem cell. Asian-Aust J Anim Sci. 2009;22:187–93.
Yang Y, Yarahmadi M, Honaramooz A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod Fert Develop. 2010;22:1057–65.
Chang Y, Jennifer S. Lee-Chang, Panneerdoss S, MacLeanII JA, Rao MK. Isolation of sertoli, Leydig, and spermatogenic cells from the mouse testis. Biotechniques. 2011;51:341–4.
Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012;29:1029–38.
Creemers LB, den Ouden K, van Pelt AMM, de Rooij DG. Maintenance of adult mouse type a spermatogonia in vitro: influence of serum and growth factors and comparison with prepubertal spermatogonial cell culture. Reproduction. 2002;124:791–9.
Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–30.
Sousa M, Cremades N, Alves C, Silva J, Barros A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum Reprod. 2002;17:161–72.
van der Wee K, Johnson EW, Dirami G, Dym M, Hofmann MC. Immunomagnetic isolation and long term culture of mouse type a spermatogonia. J Androl. 2001;22:696–704.
Zhang DY, He DW, Wei GH, Song XF, Li XL, In T. Long-term coculture of spermatogonial stem cells on sertoli cells feeder layer in vitro, Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:6-9
Kurita K, Sakai N. Functionally distinctive testicular cell lines of zebra fish to support male germ cell development. Mol Reprod Dev. 2004;67:430–8.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari TZ, Azizi H, Hosseini SE, Vahdati A, Baharvand H, Nasr-Esfahani M.H. Comparative Immunohistochemical Analysis of VASA, PLZF and THY1 in Goats and Sheep Suggests that these Markers are also Conserved in these Species. J Cytol Histol. 2011;2.
Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504-5509.
He Z, Kokkinaki M, Dym M. Signaling molecules and pathways regulating the fate of spermatogonial stem cells. Microsc Res Tech. 2009;72:586–95.
Franke WW, Schmid E, Schiller DL, Winter S, Jarasch ED, Moll R, et al. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46:431–53.
Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37.
Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, et al. Homing of mouse spermatogonial stem cells to germline niche depends on β1 integrin. Cell Stem Cell. 2008;3:533–42.
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–12.
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–9.
Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–49.
Acknowledgments
This work is supported by National Fellow project, Indian Council of Agricultural Research (ICAR), Government of India. Authors duly acknowledge ICAR-Senior Research Fellowship to RKP during his PhD programme and Dr. G.K. Das, Principal Scientist, Animal Reproduction Division, IVRI for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The results of this study demonstrated a simple three-step enzymatic digestion method for isolation, a Percoll density gradient method for enrichment and the use of Sertoli cell feeder layer for long term culture of SSC in goat.
Rights and permissions
About this article
Cite this article
Pramod, R.K., Mitra, A. In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J Assist Reprod Genet 31, 993–1001 (2014). https://doi.org/10.1007/s10815-014-0277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0277-1