Abstract
Purpose
Non-obstructive azoospermia (NOA) is one the many causes of male infertility (10 %) resulting from testicular failure. Multiple testicular biopsies fail to find mature sperm in at least 50 % of cases Therefore; hunting for sensitive and specific biomarkers of spermatogenesis that could better determine the fertility status in NOA can lead to improved management of male infertility. Therefore, we evaluated sperm production through analyses of germ cell-specific transcripts (DAZ, TSPY1, SPTRX3 and SPTRX1) in semen and testicular biopsies of men with azoospermia.
Methods
We collected semen (N = 83) and testis biopsies (N = 31) from men with non-obstructive azoospermia. We later extracted RNA and synthesized cDNA using washed semen precipitate and testicular tissues. We also performed semi-nested PCR with designed specific primers. Using H&E method, an expert pathologist performed the histopathological evaluation. Having categorized the patients into three groups based on histopathological results, we calculated the agreement between molecular results of semen and tissues with histopathological findings for each patient using Kappa statistical test.
Results
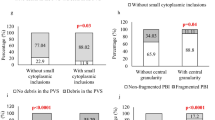
Molecular findings of precipitated semen and testicular tissues were in disagreement with histopathological results in most cases. Molecular analysis of testis biopsies showed significant difference (Kappa coefficient = 0.009, P value = 0.894) with histopathological results; TSPY1, DAZ, SPTRX3 and SPTRX1 were respectively detected in 94 %, 94 %, 17.6 % and 52.9 % of men diagnosed with germ cell aplasia.
Conclusions
Molecular analysis of semen does not provide sufficient sensitivity and specificity to be used as a screening test at the present time, but it is a useful adjunct to histopathological methods in men with NOA. Spermatid/sperm specific transcripts indicated the possibility to find mature sperm following repeated multiple testicular sperm extraction (TESE) or microdisection TESE (mTESE).



Similar content being viewed by others
References
Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum Reprod Update. 2007;13(6):539.
Matsumiya K, Namiki M, Takahara S, Kondoh N, Takada S, Kiyohara H, et al. Clinical study of azoospermia. Int J Androl. 1994;17(3):140–2.
Pantke P, Diemer T, Marconi M, Bergmann M, Steger K, Schuppe HC, et al. Testicular sperm retrieval in azoospermic men. Eur Urol Suppl. 2008;7(12):703–14.
Paz G, Gamzu R, Yavetz H. Diagnosis of nonobstructive azoospermia: the laboratory perspective. J Androl. 2003;24(2):167.
Marconi M, Keudel A, Diemer T, Bergmann M, Steger K, Schuppe HC et al. Combined trifocal and microsurgical testicular sperm extraction is the best technique for testicular sperm retrieval in “Low-Chance” nonobstructive Azoospermia. Eur Urol. 2012.
Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. J Androl. 2011.
Mercan R, Urman B, Alatas C, Aksoy S, Nuhoglu A, Isiklar A, et al. Outcome of testicular sperm retrieval procedures in non-obstructive azoospermia: percutaneous aspiration versus open biopsy. Hum Reprod. 2000;15(7):1548–51.
Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol. 2003;1:107.
Fedder J. Nonsperm cells in human semen: with special reference to seminal leukocytes and their possible influence on fertility. Syst Biol Reprod Med. 1996;36(1):41–65.
Ariagno J, Curi S, Mendeluk G, Grinspon D, Repetto H, Chenlo P, et al. Shedding of immature germ cells. Syst Biol Reprod Med. 2002;48(2):127–31.
Lau YFC, Li Y, Kido T. Role of the Y-located putative gonadoblastoma gene in human spermatogenesis. Syst Biol Reprod Med. 2011;27–34.
Schnieders F, Dörk T, Arnemann J, Vogel T, Werner M, Schmidtke J. Testis-specific protein, Y-encoded (TSPY) expression in testicular tissues. Hum Mol Genet. 1996;5(11):1801.
Jiménez A, Zu W, Rawe VY, Pelto-Huikko M, Flickinger CJ, Sutovsky P, et al. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem. 2004;279(33):34971.
Miranda-Vizuete A, Ljung J, Damdimopoulos AE, Gustafsson JÅ, Oko R, Pelto-Huikko M, et al. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem. 2001;276(34):31567.
Yen PH. Putative biological functions of the DAZ family. Int J Androl. 2004;27(3):125–9.
Szczerba A, Jankowska A, Andrusiewicz M, Karczewski M, Turkiewicz W, Warchol J. Distribution of the DAZ gene transcripts in human testis. Folia Histochem Cytobiol. 2004;42(2):119–22.
Organization WH. WHO laboratory manual for the examination and processing of human semen. World Health Organ. 2010.
Aslani F, Modarresi MH, Soltanghoraee H, Akhondi MM, Shabani A, Lakpour N et al. Seminal molecular markers as a non-invasive diagnostic tool for the evaluation of spermatogenesis in non-obstructive azoospermia. Syst Biol Reprod Med. 2011;1–7.
Aarabi M, Modarressi MH, Soltanghoraee H, Behjati R, Amirjannati N, Akhondi MM. Testicular expression of synaptonemal complex protein 3 (SYCP3) messenger ribonucleic acid in 110 patients with nonobstructive azoospermia. Fertil Steril. 2006;86(2):325–31.
Song GJ, Lee HS, Park YS, Lee HJ, Lee YS, Seo JT, et al. Expression pattern of germ cell-specific genes in the testis of patients with nonobstructive azoospermia: usefulness as a molecular marker to predict the presence of testicular sperm. Fertil Steril. 2000;73(6):1104–8.
Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod. 1993;8(7):1055.
Cerilli LA, Kuang W, Rogers D. A practical approach to testicular biopsy interpretation for male infertility. Arch Pathol Lab Med. 2010;134(8):1197–204.
Lee JH, Lee DR, Yoon SJ, Chai YG, Roh SI, Yoon HS. Expression of DAZ (deleted in azoospermia), DAZL1 (DAZ-like) and protamine-2 in testis and its application for diagnosis of spermatogenesis in non-obstructive azoospermia. Mol Hum Reprod. 1998;4(9):827.
Levek-Motola N, Soffer Y, Shochat L, Raziel A, Lewin L, Golan R. Flow cytometry of human semen: a preliminary study of a non-invasive method for the detection of spermatogenetic defects. Hum Reprod. 2005;20(12):3469.
Yeung C, Beiglböck Karau L, Tüttelmann F, Nieschlag E. The presence of germ cells in the semen of azoospermic, cryptozoospermic and severe oligozoospermic patients: stringent flow cytometric analysis and correlations with hormonal status. Clin Endocrinol. 2007;67(5):767–75.
Yeung C, Beiglböck Karau L, Luetjens C, Wunsch A, Nieschlag E. Quantification of seminal germ cells in azoospermia: correlations with testicular histology and TESE outcome. Int J Androl. 2009;32(3):242–54.
Li H, Wu C, Gu X, Xiong C. A novel application of cell-free seminal mRNA: non-invasive identification of the presence of germ cells or complete obstruction in men with azoospermia. Hum Reprod. 2012.
Wu W, Hu Z, Qin Y, Dong J, Dai J, Lu C, et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol Hum Reprod. 2012;18(10):489–97.
Rolland A, Lavigne R, Dauly C, Calvel P, Kervarrec C, Freour T, et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod. 2013;28(1):199–209.
Lundin K, Söderlund B, Hamberger L. The relationship between sperm morphology and rates of fertilization, pregnancy and spontaneous abortion in an in-vitro fertilization/intracytoplasmic sperm injection programme. Hum Reprod. 1997;12(12):2676–81.
Acknowledgments
This study was financially supported by grant No. 1424-33 from the Academic Center for Education, Culture and Research (ACECR) in Tehran, Iran. We would like to thank Ms. Elham Savadi Shirazi for helping with the sample collection and Mr. Iman Derakhsh for the artwork preparation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule The molecular analysis of testicular tissues and semen precipitates for the presence of germ cell-specific transcripts is a diagnostic test that could be of help in the detection of active spermatogenesis in men with non-obstructive azoospermia.
Rights and permissions
About this article
Cite this article
Eghbali, M., Sadeghi, M.R., Lakpour, N. et al. Molecular analysis of testis biopsy and semen pellet as complementary methods with histopathological analysis of testis in non-obstructive azoospermia. J Assist Reprod Genet 31, 707–715 (2014). https://doi.org/10.1007/s10815-014-0220-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0220-5