Abstract
Purpose
The arrangement of the blastomeres within the 4-cell stage embryo reflects the orientation of the cleavage planes during the second division. To examine their relevance, the developmental capacity and the pregnancy rate were compared between tetrahedral-shaped and non-tetrahedral-shaped 4-cell stage human embryos.
Methods
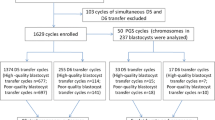
The study included 3,546 4-cell stage embryos. The arrangement of the blastomeres at the 4-cell stage was annotated as being tetrahedral or non-tetrahedral on day 2 of preimplantation development. Embryo quality was compared on day 3 and day 5. Pregnancy rates were calculated per single embryo transfer on day 3 or day 5.
Results
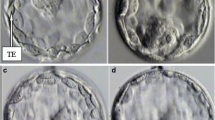
In total, 2,803 4-cell stage embryos (79 %) displayed a tetrahedral arrangement and 743 (21 %) displayed a non-tetrahedral arrangement. Tetrahedral-shaped embryos developed more into high-quality embryos on day 3 (p < 0.001) and day 5 (p = 0.036) and had a higher blastulation rate (p = 0.009). Though, the number of high-quality embryos selected for transfer did not differ between both groups on day 3 (p = 0.167) and day 5 (p ~ 1). Three hundred thirty single embryo transfers were analysed. No significant difference in clinical pregnancy was found between both groups after transfer on day 3 (p = 0.209) and day 5 (p = 0.653).
Conclusions
The arrangement of the blastomeres according to their previous cleavage planes was correlated to the developmental potential of the 4-cell stage embryo up to the blastocyst stage. If embryo transfers are performed on day 3 and day 5 of development using embryos of adequate quality, the blastomere arrangement at the 4-cell stage had no predictable value regarding pregnancy success.


Similar content being viewed by others
References
Gulyas BJ. A reexamination of cleavage patterns in eutherian mammalian eggs: rotation of blastomere pairs during second cleavage in the rabbit. J Exp Zool. 1975;193:235–48.
Gilbert SF. Developmental biology. 5th ed. Sunderland: Sinauer Associates; 1997.
Edwards RG, Steptoe PC, Purdy JM. Fertilization and cleavage in vitro of preovulatory human oocytes. Nature. 1970;227:1307–9.
Gardner RL. Experimental analysis of second cleavage in the mouse. Hum Reprod. 2002;12:3178–89.
Edwards RG, Beard HK. Oocyte polarity and cell determination in early mammalian embryos. Mol Hum Reprod. 1997;3:863–905.
Antczak M, Van Blerkom J. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod. 1997;3:1067–86.
Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14:429–47.
Edwards RG. Genetics of polarity in mammalian embryos. Reprod Biomed Online. 2005;11:104–14.
Howlett SK, Bolton VN. Sequence and regulation of morphological and molecular events during the first cycle of mouse embryogenesis. J Embryol Exp Morphol. 1985;87:175–206.
Payne D, Adachi D, Kato Y, Ueno Y, Mio Y, Flaherty S. Polarity and early human embryo development. Fertil Steril. 2004;82:S7.
Edwards RG, Hansis C. Initial differentiation of blastomeres in 4-cell human embryos and its significance for early embryogenesis and implantation. Reprod Biomed Online. 2005;11:206–18.
Hansis C, Grifo JA, Krey LC. Candidate lineage marker genes in human preimplantation embryos. Reprod Biomed Online. 2004;8:577–83.
Nagy ZP, Liu J, Joris H, Bocken G, Desmet B, Van Ranst H, et al. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection on fertilization and embryo development rates. Hum Reprod. 1995;10:3171–7.
Johnson MH. From mouse egg to mouse embryo: polarities, axes, and tissues. Annu Rev Cell Dev Biol. 2009;25:483–512.
Mastenbroek S, van der Veen F, Aflatoonian A, Shapiro B, Bossuyt P, Repping S. Embryo selection in IVF. Hum Reprod. 2011;26:964–6.
Ajduk A, Zernicka-Goetz M. Advances in embryo selection methods. F1000 Biol Rep. 2012;4:11.
Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–40.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Prados F, Debrock S, Lemmen J, Agerholm A. The cleavage stage embryo. Hum Reprod. 2012;27:i50–71.
Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–8.
Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod. 2008;14:703–10.
Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–66.
Wong C, Chen AA, Behr B, Shen S. Time-lapse microscopy and image analysis in basic and clinical embryo development research. Reprod Biomed Online. 2013;26:120–9.
Hardarson T, Ahlström A, Rogberg L, Botros L, Hillensjö T, Westlander G, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27:89–96.
Vergouw CG, Kieslinger DC, Kostelijk EH, Botros LL, Schats R, Hompes PG, et al. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27:2304–11.
Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 2011;32:S257–63.
Papanikolaou EG, D’haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod. 2005;20:3198–203.
Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update. 2003;9:251–62.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. London: Parthenon Publishing; 1999. p. 378–88.
Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirtheghem A. Blastocyst formation in in vitro versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83:1397–403.
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24:2683–7.
Piotrowska-Nitsche K, Zernicka-Goetz M. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev. 2005;122:487–500.
Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23:62–6.
Nikas G, Ao A, Winston RM, Handyside AH. Compaction and surface polarity in the human embryo in vitro. Biol Reprod. 1996;55:32–7.
Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10:1571–8.
Bloor DJ, Metcalfe AD, Rutherford A, Brison DR, Kimber SJ. Expression of cell adhesion molecules during human preimplantation embryo development. Mol Hum Reprod. 2002;8:237–45.
Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod. 2005;20:3369–75.
Paternot G, Spiessens M, Verstreken D, Van Bauwel J, Debrock S, D’Hooghe T, et al. Is there a link between blastomere contact surfaces of day 3 embryos and live birth rate? Reprod Biol Endocrinol. 2012;10:78.
Kamran SC, Reichman DE, Missmer SA, Correia KF, Karaca N, Romano A, et al. Day 3 embryo shape as a morphologic selection parameter in in vitro fertilization. J Assist Reprod Genet. 2012;29:1135–9.
Ebner T, Maurer M, Shebl O, Moser M, Mayer RB, Duba HC, et al. Planar embryos have poor prognosis in terms of blastocyst formation and implantation. Reprod Biomed Online. 2012;25:267–72.
Munné S. Chromosome abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online. 2005;12:234–53.
Van de Velde H, Cauffman G, Tournaye H, Devroey P, Liebaers I. The four blastomeres of a 4-cell stage human embryo are able to develop individually into blastocysts with inner cell mass and trophectoderm. Hum Reprod. 2008;23:1742–7.
Mottla GL, Adelman MR, Hall JL, Gindoff PR, Stillman RJ, Johnson KE. Lineage tracing demonstrates that blastomeres of early cleavage-stage human pre-embryos contribute to both trophectoderm and inner cell mass. Hum Reprod. 1995;10:384–91.
Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132:479–90.
Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445:214–8.
Torres-Padilla ME. Cell identity in the preimplantation mammalian embryo: an epigenetic perspective from the mouse. Hum Reprod. 2008;23:1246–52.
Hiiragi T, Solter D. First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature. 2004;430:360–4.
Zheng JG, Huo T, Chen T, Wang C, Zhang N, Tian N, et al. Understanding three-dimensional spatial relationship between the mouse second polar body and first cleavage plane with full-field optical coherence tomography. J Biomed Opt. 2013;18:10503.
Louvet-Vallée S, Vinot S, Maro B. Mitotic spindles and cleavage planes are oriented randomly in the two-cell mouse embryo. Curr Biol. 2005;15:464–9.
Acknowledgments
The authors wish to thank the laboratory staff of the Centre for Reproductive Medicine, UZ Brussel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Tetrahedral-shaped 4-cell stage human embryos have a better developmental capacity up to the blastocyst stage compared to non-tetrahedral-shaped embryos.
Rights and permissions
About this article
Cite this article
Cauffman, G., Verheyen, G., Tournaye, H. et al. Developmental capacity and pregnancy rate of tetrahedral- versus non-tetrahedral-shaped 4-cell stage human embryos. J Assist Reprod Genet 31, 427–434 (2014). https://doi.org/10.1007/s10815-014-0185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0185-4