Abstract
Purpose
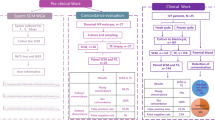
Only 30 % of IVF cycles result in a pregnancy, so that multiple embryos need to be replaced, per treatment cycle, to increase pregnancy rates, resulting in a multiple gestation rate of 25 %. The use of new markers in the gamete selection, could reduce the number of the oocytes to be fertilized and embryos to be produced, but the tools to evidence the gamete competence remain unavailable and more studies are needed to identify bio-markers to select the best oocyte and sperm to produce embryos with higher implantation potentiality.
Methods
To define oocyte competence, the apoptosis of the surrounding cumulus cells and the oxygen consumption rates for individual oocytes before fertilization seems to provide a non-invasive marker of oocyte competence and hence a quantitative assessment of the reproductive potential for the oocyte. The chromatin integrity seems to be used also as biological marker of sperm competence, together with the morphological evaluation of large vacuoles in the head.
Results
The apoptosis rate of cumulus cells lower than 25 % and an higher oxygen consumption could be an evidence of an overall metabolic activity, related to a better fertilization ability and embryo cleavage quality. The apoptosis rate of the sperm chromatin, evaluated by direct Tunel in situ analysis, seems to be, also for the male gamete, a marker of competence and implantation potentiality, in particular when it is lower than 20 %. The evaluation of the presence of large vacuoles in the sperm head prior to perform ICSI seems to increase the implantation rate, but it is not associated to chromatin integrity.
Conclusions
The biological concept of competence appears unrelated to any morphological parameters, so that it is necessary to investigate new molecular markers in the gamete selection. Apoptosis of cumulus cells in the oocytes and spermatozoa, revealing the presence of large vacuoles, could help to determine the competence of the gamete to be fertilize.
Similar content being viewed by others
References
De Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al. Assisted reproductive technology in Europe, 2006: resultsgenerated fromEuropean registers by ESHRE. Hum Reprod. 2010;25:1851–62.
Eppig JJ, Schultz RM, O’Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9.
Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann N Y Acad Sci. 2004;1034:132–44. Review.
Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19:1–12. Review.
Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–70.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3.
Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, et al. Identification of potential markers of oocyte competence expressed in bovine. cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod. 2008;79:209–22.
Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99(5):2890–4. Epub 2002 Feb 26.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–80. Review.
Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–46.
Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, et al. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996;11:1480–3.
Bosco L, Ruvolo G, Morici G, Manno M, Cittadini E, Roccheri MC. Apoptosis in human unfertilized oocytes after intracytoplasmic sperm injection. Fertil Steril. 2005;84(5):1417–23.
Huang JY, Chen HY, Park JY, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril. 2008;90(4 Suppl):1424–32. Epub 2007 Oct 24.
Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936–42.
Host E, Mikkelsen AL, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to maturation stage and cleavage of the corresponding oocyte. Acta Obstet Gynecol Scand. 2000;79:936–40.
Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril. 2007;87(3):542–6.
Carabatos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9- deficient mice. Dev Biol. 1998;204:373–84.
Joyce IM, Clark AT, Pendola FL, Eppig JJ. Comparison of GDF-9 and oocyte regulation of kit ligand expression in mouse ovarian follicles. Biol Reprod. 2000;63:1669–75.
Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–48.
Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of Kit ligand expression in mouse ovarian follicles. Dev Biol. 2001;214:342–53.
Tajima K, Orisaka M, Hosokawa K, Amsterdam A, Kotsuji F. Effects of ovarian theca cells on apoptosis and proliferation of granulosa cells: chancing during bovine follicular maturation. Biol Reprod. 2002;66:1635–9.
Harper KM, Brackett BG. Bovine blastocyst development after in vitromaturation in a defined medium with epidermal growth factor andlow concentrations of gonadotropins. Biol Reprod. 1993;48:409–16.
Ding J, Foxcroft GR. Epidermal growth factor enhances oocyte maturationin pigs. Mol Reprod Dev. 1994;39:30–40.
Dekel N, Sherizly I. Epidermal growth factor induces maturation of ratfollicle-enclosed oocytes. Endocrinology. 1985;116:406–9.
Das K, Stout LE, Hensleigh HC, Tagatz GE, Phipps WR, Leung BS. Directpositive effect of epidermal growth factor on the cytoplasmic maturationof mouse and human oocytes. Fertil Steril. 1991;55:1000–4.
Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri. A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84.
Hardy K, Spanos S. Growth factor expression and function in the humanand mouse preimplantation embryo. J Endocrinol. 2002;172:221–36.
Ben-Ami I, Armon L, Freimann S, Strassburger D, Ron-El R, Amsterdam A. EGF-like growth factors as LH mediators in the human corpus luteum. Hum Reprod. 2009;24(1):176–84. Epub 2008 Oct 3.
Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292(1):F440–7. Epub 2006 Aug 1.
Jie B, Zhang X, Wu X, Xin Y, Liu Y, Guo Y. Neuregulin-1 suppresses cardiomyocyte apoptosis by activating PI3K/Akt and inhibiting mitochondrial permeability transition pore. Mol Cell Biochem. 2012;370(1–2):35–43.
Tejera A, Herrero J, de los Santos MJ, Garrido N, Ramsing N, Meseguer M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil Steril. 2011;96(3):618.e2–23.e2.
O’Shea LC, Mehta J, Lonergan P, Hensey C, Fair T. Developmental competence in oocytes and cumulus cells: candidate genes and networks. Syst Biol Reprod Med. 2012;58(2):88–101.
Bartoov B, Eltes F, Pansky M, Lederman H, Caspi E, Soffer Y. Estimating fertility potential via semen analysis data. Hum Reprod. 1993;8(1):65–70.
Berkovitz A, Eltes F, Soffer Y, Zabludovsky N, Beyth Y, Farhi J, et al. ART success and in vivo sperm cell selection depend on the ultra-morphological status of spermatozoa. Andrologia. 1999;31(1):1–8.
Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49(1):112–7.
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174–9. Epub 2006 Aug 18.
Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. National Cooperative Reproductive Medicine Network. N Engl J Med. 2001;345(19):1388–93.
Marchetti F, Essers J, Kanaar R, Wyrobek AJ. Disruption of maternalDNA repair increases sperm-derived chromosomal aberrations. Proc Natl AcadSci U S A. 2007;104:17725–9.
Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility ofthe sperm chromatin structure assay as a diagnostic and prognostic tool in thehuman fertility clinic. Hum Reprod. 1999;14:1039–49.
Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI:systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8.
Frydman N, Prisant N, Hesters L, Frydman R, Tachdjian G, Cohen-Bacrie P, et al. Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil Steril. 2008;89(1):92–7. Epub 2007 May 4.
Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69(3):528–32.
Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic Trans generational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–9.
Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21(7):1787–90. Epub 2006 Feb 23.
Franco Jr JG, Mauri AL, Petersen CG, Massaro FC, Silva LF, Felipe V, et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl. 2012;35(1):46–51. Epub 2011 Apr 28.
Falagario D, Brucculeri AM,Depalo R, Trerotoli P, Cittadini E, Ruvolo G. Sperm head vacuolization affects clinical outcome in ICSI cycle. A proposal of a cut-off value. J Assist Reprod Genet. 2012; E pub Sep 12.
García-Alvarez O, Maroto-Morales A, Ramón M, del Olmo E, Montoro V, Dominguez-Rebolledo AE, et al. Analysis of selected sperm by density gradient centrifugation might aid in the estimation of in vivo fertility of thawed ram spermatozoa. Theriogenology. 2010;74(6):979–88. Epub 2010 Jun 26.
Magli MC, Crippa A, Muzii L, Boudjema E, Capoti A, Scaravelli G, et al. Head birefringence properties are associated with acrosome reaction, sperm motility and morphology. Reprod Biomed Online. 2012;24(3):352–9. Epub 2012 Jan 8.
Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18(3):260–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule To identify bio-markers to select the best oocyte and sperm to produce embryos with higher implantation potentiality, is one of the main interests of the researchers. Cumulus cells apoptosis, oocyte oxygen consumption, vacuolization of sperm head and chromatin integrity seems to be a promising tools.
Rights and permissions
About this article
Cite this article
Ruvolo, G., Fattouh, R.R., Bosco, L. et al. New molecular markers for the evaluation of gamete quality. J Assist Reprod Genet 30, 207–212 (2013). https://doi.org/10.1007/s10815-013-9943-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-9943-y