Abstract
Purpose
To determine expression of G-protein estrogen receptor (GPER) in mouse oocyte membrane during maturation.
Methods
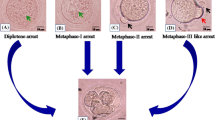
The expression of GPER from different maturation stages of oocytes, in vivo and in vitro matured oocytes as well as aging oocytes was examined by immune-fluorescence GPR30 antibody and the images were analyzed by laser scanning confocal microscope. Further confirmation was performed by Western blots for cell fractionation.
Results
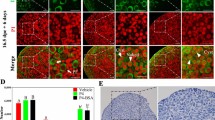
Significant fluorescent signal was observed on the surface of mouse oocytes. The image expression was lower in germinal vesicle (GV) stage than mature metaphase-II (M-II) stage oocytes. There was high expression in in-vivo matured oocytes compared to in vitro matured oocytes. The highest expression was observed in aging oocytes compared with other oocytes.
Conclusions
The changes of expression of GPER on mouse oocytes plasma membrane confirm oocyte membrane maturation, suggesting that those changes of GPER may be related to the functional role of oocyte maturation.




Similar content being viewed by others
References
Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–26.
Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602.
Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–91.
Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–302.
Rettew JA, McCall SHT, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328:87–92.
Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–8.
Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–82.
Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60.
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30.
Thomas D, Kim HY, Hanley MR. Regulation of inositol trisphosphate-induced membrane currents in Xenopus oocytes by a Jurkat cell calcium influx factor. Biochem J. 1996;318(Pt 2):649–56.
Thomas D, Kim HY, Morgan R, Hanley MR. Double-stranded-RNA-activated protein kinase (PKR) regulates Ca2+ stores in Xenopus oocytes. Biochem J. 1998;330(Pt 2):599–603.
Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32.
Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265–266:138–42.
Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–96.
Ge C, Yu M, Zhang C. G protein-coupled receptor 30 mediates estrogen-induced proliferation of primordial germ cells via EGFR/Akt/beta-catenin signaling pathway. Endocrinology. 2012;153:3504–16.
Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod Biomed Online. 2004;8:148–66.
Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio). Biol Reprod. 2011;85:42–50.
Quinn PJ, Takahashi H, Hatta I. Characterization of complexes formed in fully hydrated dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys J. 1995;68:1374–82.
Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50 Suppl 1:S195–219.
Mishra A, Joy KP. Involvement of mitogen-activated protein kinase in 2-hydroxyestradiol-17beta-induced oocyte maturation in the catfish Heteropneustes fossilis and a note on possible interaction with protein phosphatases. Gen Comp Endocrinol. 2006;147:329–35.
Fan HY, Sun QY. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod. 2004;70:535–47.
Gallo CJ, Hand AR, Jones TL, Jaffe LA. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein alpha S subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130:275–84.
Moody WJ, Lansman JB. Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc Natl Acad Sci U S A. 1983;80:3096–100.
Tesarik J, Sousa M. Comparison of Ca2+ responses in human oocytes fertilized by subzonal insemination and by intracytoplasmic sperm injection. Fertil Steril. 1994;62:1197–204.
Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, et al. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–65.
Toranzo GS, Buhler MC, Buhler MI. Participation of IP3R, RyR and L-type Ca2+ channel in the nuclear maturation of Rhinella arenarum oocytes. Zygote 2012:1–14.
Tosti E. Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol. 2006;4:26.
Carroll J, Jones KT, Whittingham DG. Ca2+ release and the development of Ca2+ release mechanisms during oocyte maturation: a prelude to fertilization. Rev Reprod. 1996;1:137–43.
Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84.
Kostellow AB, Ziegler D, Morrill GA. Regulation of Ca2+ and cyclic AMP during the first meiotic division in amphibian oocytes by progesterone. J Cyclic Nucleotide Res. 1980;6:347–58.
Silvestre F, Boni R, Fissore RA, Tosti E. Ca2+ signaling during maturation of cumulus-oocyte complex in mammals. Mol Reprod Dev. 2011;78:744–56.
Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–91.
Huang JY, Chen HY, Park JY, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril. 2008;90:1424–32.
Son WY, Chung JT, Demirtas E, Holzer H, Sylvestre C, Buckett W, et al. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod Biomed Online. 2008;17:59–67.
Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27:456–64.
Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet. 2004;21:233–40.
Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–98.
Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–30.
Whitaker MJ, Swann K. Lighting the fuse at fertilization. Development. 1993;117:1–12.
Herbert M, Gillespie JI, Murdoch AP. Development of calcium signalling mechanisms during maturation of human oocytes. Mol Hum Reprod. 1997;3:965–73.
Ma SF, Liu XY, Miao DQ, Han ZB, Zhang X, Miao YL, et al. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology. 2005;64:1142–57.
Acknowledgments
This research was supported by MUHC Reproductive Center and a grant from the China Natural Science Foundation (81270746) to RCC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule
The changes of expression of GPER on mouse oocytes plasma membrane confirm “oocyte membrane maturation”
Rights and permissions
About this article
Cite this article
Li, YR., Ren, CE., Zhang, Q. et al. Expression of G protein estrogen receptor (GPER) on membrane of mouse oocytes during maturation. J Assist Reprod Genet 30, 227–232 (2013). https://doi.org/10.1007/s10815-013-9942-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-9942-z