Abstract
Purpose
To explore the effects of controlled ovarian stimulation (COS) on the expression of growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) in oocytes and granulosa cells from patients with or without polycystic ovary syndrome (PCOS).
Methods
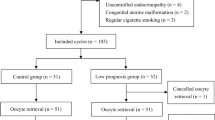
This case–control study was conducted in the university affiliated hospital. The study comprised four groups of patients: eighteen PCOS patients with COS (stimulated-PCOS) and twenty-two PCOS patients without COS (unstimulated-PCOS), twenty-nine normal ovulatory women with COS (stimulated-control) and twenty-eight normal ovulatory women without COS (unstimulated-control). The oocytes and granulosa cells were collected and the abundance of GDF9 and BMP15 mRNA in the cells were detected by nested quantitative real-time PCR.
Results
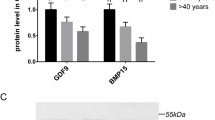
The abundance of GDF9 and BMP15 mRNA was significantly higher both in oocytes (P < 0.01, P < 0.001, respectively) and GCs (P < 0.01, P < 0.05, respectively) from stimulated-control group than in unstimulated-control group. However, there was no significant difference for GDF9 or BMP15 mRNA in oocytes from stimulated-PCOS goup compared with unstimulated-PCOS group (P > 0.05, P > 0.05, respectively). The abundance of GDF9 mRNA was significantly lower (P < 0.01) while the abundance of BMP15 mRNA was significantly higher (P < 0.001) in GCs from stimulated-PCOS group than in unstimulated-PCOS group.
Conclusions
The controlled ovarian stimulation can promote the expression of GDF9 and BMP15 both in oocytes and GCs from normal ovulatory women. However, the stimulating effects may be inhibited in oocytes from PCOS patients, which subsequently impair cytoplasm maturation and lead to poor oocyte quality.
Similar content being viewed by others
References
Teede H, Deeks A, Moran L. Polycystc ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;30:41.
Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405.
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51.
Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21.
Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33.
Juengel JL, Mc Natty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11:143–60.
Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206.
Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77.
Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online. 2007;14:758–64.
Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–66.
Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–21.
Yeo CX, Gilchrist RB, Thompson JG, Lane M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23:67–73.
The Rotterdam ESHRE/ASRM sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod. 2010;83:514–24.
Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–6.
Desforges-Bullet V, Gallo C, Lefebvre C, Pigny P, Dewailly D, Catteau-Jonard S. Increased anti-Müllerian hormone and decreased FSH levels in follicular fluid obtained in women with polycystic ovaries at the time of follicle puncture for in vitro fertilization. Fertil Steril. 2010;94:198–204.
Grossman MP, Nakajima ST, Fallat ME, et al. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2007;89(5 Suppl):1364–70.
Chang HM, Klausen C, Leung PC. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013. doi:10.1016/j.fertnstert.2013.04.019.
Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–78.
Adriaenssens T, Wathlet S, Segers I, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–70.
Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16:531–8.
Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16:715–25.
Acknowledgments
We gratefully acknowledged the generous supports of the National Natural Science Foundation of China (Grant No.81200476), National Doctoral Foundation of China (Grant No. 20120171120122), Natural Science Foundation of Guangdong Province (Grant No. S2012040007770), Medical Science and Technology Research Foundation of Guangdong Province (Grant No. B2012150).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Inhibited expression of OSFs in PCOS oocytes.
Rights and permissions
About this article
Cite this article
Wei, LN., Li, LL., Fang, C. et al. Inhibitory effects of controlled ovarian stimulation on the expression of GDF9 and BMP15 in oocytes from women with PCOS. J Assist Reprod Genet 30, 1313–1318 (2013). https://doi.org/10.1007/s10815-013-0041-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-0041-y